INTRODUCTION
Retaining spermatozoa viability is a key aim in successful cryopreservation. Ram semen is prone to biological and functional damage at freezing temperatures (2). Spermatozoa cell membrane, as any other cell in the body, has polyunsaturated fatty acids which are susceptible to the process of lipid peroxidation, resulting in production of oxygen reactive species (ROS) which are the primary deteriorative agents of their biological structure (3). The membrane integrity is one of the main determinants for spermatozoa viability (5), thus impairment of its structure could cause serious decreasing of spermatozoa motility and fertilizing capacity (6-10). Furthermore, ROS can trigger early capacitation or hyper-motility of spermatozoa, increasing their concentration to a higher level (10-14). As part of the solution, some investigations report the use of anti-oxidants (oxidized glutathione - GSSG, reduced glutathione - GSH, and cysteine) as additives in the semen extenders, in an attempt to decrease the level of ROS which are inevitably produced during the freezing-thawing process of the semen (5, 9). These trials had a marked statistically significant increase in spermatozoa motility (p<0.01), but did not report any change in sperm viability, acrosome abnormality or total abnormality.
Predicting the fertility potential of spermatozoa is a key aim in any andrological investigation.
The kinetic parameters of a spermatozoon are an indicator of its capability to successfully fertilize the oocytes. In-vivo studies confirm that spermatozoa with high progressive motility and amplitude of lateral head displacement have the highest chance of migrating through the estrus cervical mucus (36-38). The computer aided sperm analyzers (CASA) give the possibility of real-time analysis of semen kinetic parameters, allowing accurate and fast results. Any change in viability, motility or structure of spermatozoa could be assigned to different groups of kinetic parameters. This gives an opportunity for the fast acquiring parameters to be used as predictive factors for spermatozoa fertilizing capability (15).
A special attribute can be given to GSH since it has the capability to concentrate in the seminal plasma (39). It’s a tripeptide compound, belonging to the group of selenocysteines, and it is related to the glutathione peroxidase enzyme which uses the GSH as a reducing equivalent for degradation of hydrogen peroxide (13). Even though it cannot pass the cell membrane, its thiolic group can directly react with hydrogen peroxide, superoxide anions and hydroxyl radicals, and its sulfhydryl group can react with alkoxyl radicals and hydroperoxides, thus producing alcohols (12). In general, it has properties of removing ROS from the seminal plasma, eliminating one of the key factors that impair spermatozoa viability. The aims of this investigation were: 1. To determine whether the presence of GSH in the soy-bean based semen extender will give higher values of kinetic parameters in thawed semen of Ovchepolian Pramenka rams; 2. To mark the kinetic parameters of thawed spermatozoa that can be positively correlated with the presence of GSH in the soy-bean based semen extender.
MATERIALS AND METHODS
Animals and semen collection
Two best-performing Ovchepolian Pramenka rams aged 3 years, were selected from the ram-flock at the Faculty of Veterinary Medicine in Skopje. The treatment and the protocols used for semen collection were in accordance with the Macedonian Law for Animal Protection and Welfare 113/07 (32). Animals were fed ad libidum with good quality alfalfa hay and 0.9 kg of concentrate per day, with free access to fresh water. They have been routinely (twice a week) included in semen collection by means of an artificial vagina. Each ram has had 2 successive mounts in one session, within an interval of 15 minutes.
The internal temperature of the artificial vagina has been set at 42-43°C by insertion of warm water (50-55°C) in the space between the inner and outer rubber. Glass-sterile collection tubes have been appropriately warmed at 35-37°C before use. The inner rubber of the artificial vagina has been lubricated with the same extender used for the semen dilution. Right after the ejaculation, the collection tubes have been isolated in thermo-insulating wrap and were rapidly transferred to the lab for initial evaluation (1).
Semen dilution, cooling and freezing
During the initial evaluation (volume, motility and sperm concentration), the semen samples were kept in a water bath at 33°C in order to lower the motility and the expenditure of metabolic energy, minimizing the conditions for production of unwanted ROS. The total volume of each ram ejaculate was split into two parts, each being included in a separate group. The first was the treatment group, which included one part of each ejaculate that would be diluted with soy-bean based extender containing GSH (5 mmol/ml). The second part of the ejaculate was diluted with the same extender that did not contain GSH additive. The initial dilution was made at a ratio of 1:1 with pre-warmed extender at temperature of 33°C. In each five consecutive minutes, an additional amount was added, equal to the initial semen volume, until reaching final concentration of around 250-300x106/ml.
The semen was packed in plastic French straws of 0.5 ml and equilibrated at 5°C for 2 hours. After equilibration the straws were put in a programmable freezing machine (IceCube 15 M, Minitüb, Tiefenbach, Germany) and frozen at rates of -5°C/minute (from 5°C to –12°C) and -60°C/minute (from – 12°C to -140°C) before being plunged into the liquid nitrogen.
Semen thawing and assessment
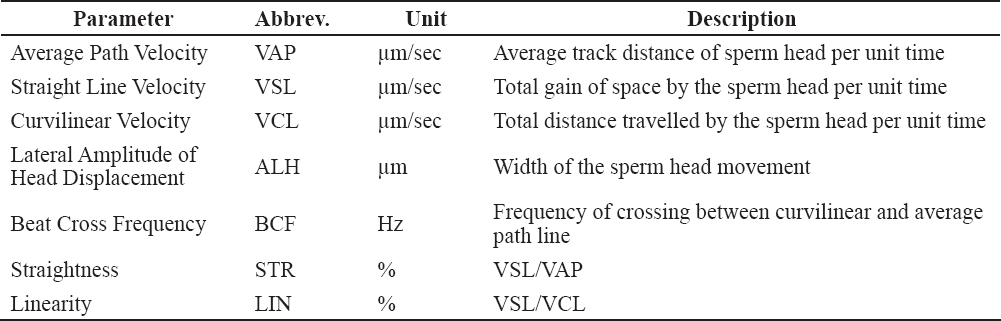
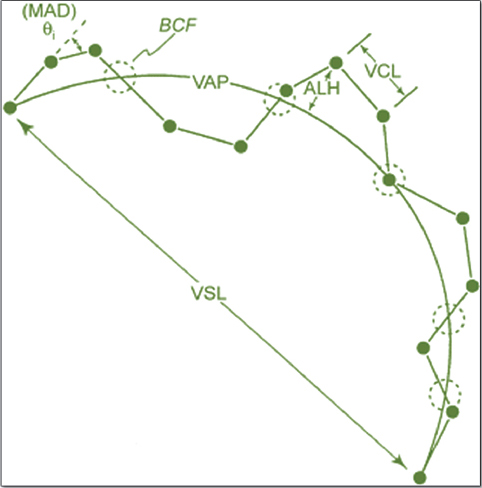
The semen samples that were the subject of the present investigation were collected in the period between 25th of January and 10th of May, 2013. To acquire the results, 48 semen samples from both groups have been thawed. The thawing procedure was performed in a water bath at 37°C for 30 seconds. The thawed semen was transferred from the French straws in pre-warmed glass tubes (35°C). They were microscopically checked for total and progressive motility before being diluted with the same extender used in the pre-thaw procedure until reaching final spermatozoa concentration of 20x106/ml. Total, progressive motility and kinetic parameters of the samples were obtained by CASA system (TOX IVOS, Hamilton Thorne Research). Four micro-liters of the diluted sample were placed on a pre-warmed counting slide (Leja 4, Leja products, Luzernestraat B. V., Holland). Each specific evaluation was performed on 10 microscopic fields to include at least 300 cells. The following CASA settings were used: Frames per second: 60 Hz; Number of frames: 30; Minimum cell size for detection: 5 pix.; Progressive cells accounted for: VAP >45 µ/s and STR >80%; Slow cells cutoff for VAP <21.9 µ/s and VSL < 6 µ/s. Table 1 presents the kinetic parameters description which were taken in consideration for this trial (17, 34). Figure 1 depicts the following parameters: VAP, VSL, VCL, ALH and BCF.
Table 1. Kinetic parameters included in the CASA assessment
Figure 1. Kinetic parameters of spermatozoon
Statistical analysis
For each group, basic descriptive statistics were used to acquire the mean value, the standard deviation (STD) and the standard error of means (SEM). The means were compared with inferential statistics model acquiring the t value. The statistical significance was considered if the probability (P) value was <0.05.
For analysis of variance, one way ANOVA model (Fisher’s test) was used to acquire the F value. The rejection of the null hypothesis was followed by a post-hock LSD test in order to determine the individual effect of each ram on the treatment group.
The correlation of variables was determined by generating a correlative matrix. The statistical significance threshold for positive and negative correlations was set at P<0.05 (StatSoft, Inc. 2005, STATISTICA, data analysis software system, version 7.1.).
RESULTS
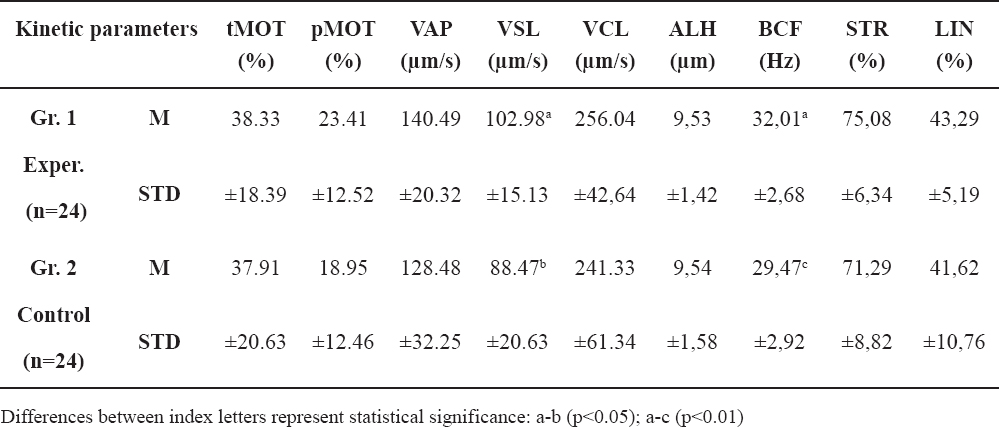
In Table 2, the means of the acquired parameters for the treatment (n=24) and control group (n=24)are presented. The results are graphically illustrated in Figures 2-8.
Table 2. Means for kinetic parameters of semen samples
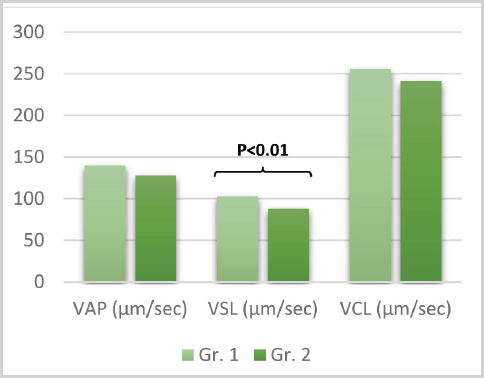
Figure 2. Means for VAP, VSL and VCL between group 1 and 2
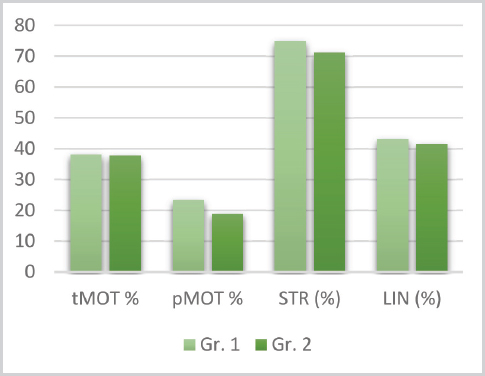
Figure 3. Means for tMOT, pMOT, STR and LIN between group 1 and 2
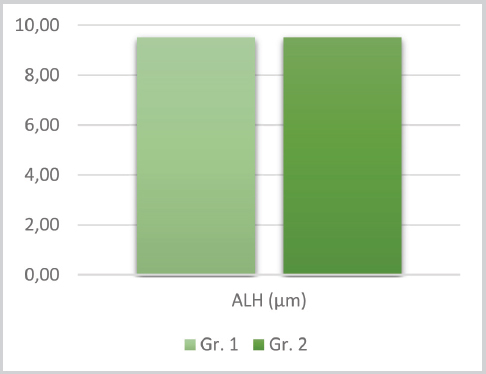
Figure 4. Means for ALH between group 1 and 2
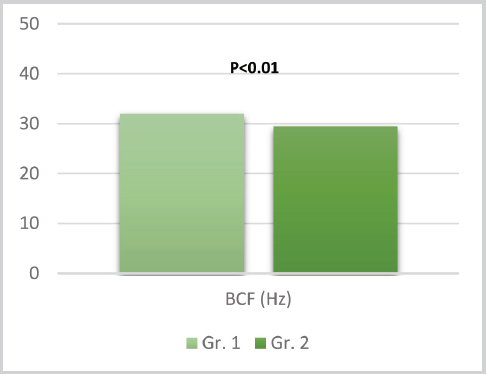
Figure 5. Means for BCF between group 1 and 2
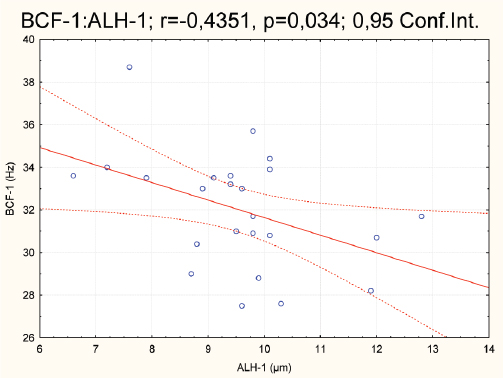
Figure 6. Correlation of BCF vs. ALH in the first group
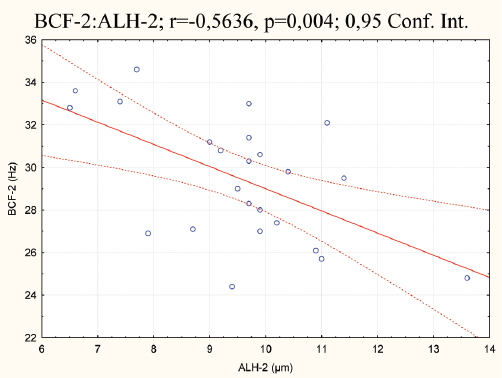
Figure 7. Correlation of BCF vs. ALH in the second group
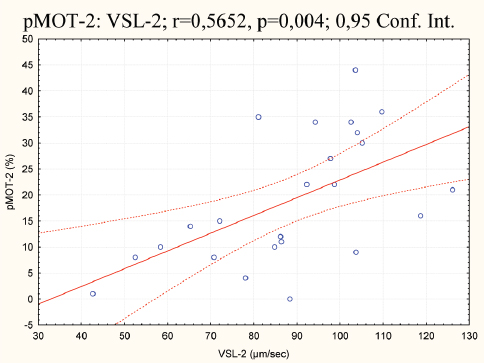
Figure 8. Correlation of pMOT and VSL in the second group
The t-test results for the tMOT, pMOT, VAP, VSL, VCL, ALH, BCF, STR, LIN between the groups (d.f.=46) are listed in the respective order: t=0.07, P=0.94; t=1.23, P=0.22; t=1.54, P=0.12; t=2.77, P=0.007; t=0.96 P=0.33; t=-0.009, P=0.99; t=3.12, P=0.003; t=1.70, P=0.09 and t=0.68, P=0.49. Significant statistical difference can be noted only for the VSL and BCF kinetic parameters (102.98±15.13 vs. 88.47±20.63 and 32.01±2.68 vs. 29.47±2.92, respectively.
Fisher’s one way variance test generated the following values in the same respective order: F=0.148, P=0.931; F=1.231, P=0.309; F=1.645, P=0.193; F=2.517, P=0.07; F=1.627, P=0.197; F=1.667, P=0.188; F=3.836, P=0.016; F=3.165, P=0.033; F=1.186, P=0.326 (d.f.=47). According to the critical (cut-off) value of 2.177 (α=0.01), null hypothesis can be rejected for the same parameters as with the previous analysis.
The LSD post-hock test which was used to determine the individual effect of ram’s ejaculates in the experimental group on the general results of the same group, showed that in the case of BCF significant impact can be attributed only to one of the rams (F=3.83 and LSD=2.76). As for the VSL, neither of the rams ejaculates showed significant influence on the experimental group (F=2.517, LSD=18.12).
With the generated correlation matrix the following r values have been acquired between the groups: r=0.42, P=0.038; r=0.32, P=0.127; r=0.16, P=0.428; r=0.28, P=0.179; r=0.11, P=0.584; r=0.61, P=0.001; r=0.27, P=0.195; r=0.15, P=0.484 and r=-0.05, P=0.783, respectively. The highest P value can be noted for ALH (r=0.61; P=0.001) and moderate for tMOT (r=0,42; P=0.038). The correlation analysis between the parameters within and between the groups points negative relationships between BCF vs. ALH, in the first (r=-0,43; P=0.034) and the second group (r=-0,56; p=0,004). A positive correlation was detected for pMOT vs. VSL, in the second group only (r=0,56; P=0.004). This is illustrated in figures 6 to 8.
DISCUSSION
In-vitro results rarely comply with the in-vivo trials when it comes to ram semen (16, 18-21). Scientific trials have attempted to utilize different assessment methods in establishing reliable correlation of in-vitro results with fertility in animals. Such trials included mitochondrial and cell membrane integrity evaluation with the artificial insemination results in rams (22) and bulls (24) or correlation of kinetic parameters of spermatozoa and their ability to migrate in cervical mucus (15-17). All these attempts have been negated by Garcia Alvarez et al. (25). Together with O’Meara et al. (26), they did not find any significant relationships between fertility and CASA results of thawed ram semen (26, 27). However, a more recent study reveals strong relationships between sperm velocity parameters (VCL, VAP, VSL and BCF) and field fertility (16), which is supported by findings of previous investigations (28-30, 35).
The present study has attempted to use the established kinetic parameters in order to make positive correlation with thawed semen samples supplemented with GSH, following conclusions from previous reports (12, 13, 31). The analysis of results regarding the variables between the groups indicate that VSL and BCF are the only parameters that are in compliance with the hypothesis. But, if individual rams are accounted in the calculations of the variables with regards to the treatment group, the negative hypothesis can be rejected only for the BCF parameter, where only one of the rams (50%) had direct influence on the results of the treatment group. The significant variance in case of VSL cannot be attributed to any of the rams, but their influence on the treatment group results is cumulative. The higher values for tMOT (1.01%) and pMOT (19.05%) in favor of the treatment group (38.30% vs. 37.91% and 23.41% vs. 18.95%, respectively) were not relevant due to very low statistical significance (P>0.05). The same conclusion can be made for VAP, VCL, STR, LIN, except ALH which had higher value for only 0,1% (P>0.05) in favor of the second group. The positive correlation between pMOT and VSL in the treatment group indicates that these values are positively affected by the presence of GSH. Similar conclusions can be found in other articles, where authors gave a different approach on this matter resulting in increase of the number of parameters with significant difference between the groups. E. Del Olmo, 2013 (16) concludes that one of the reasons for these kind of results is the insufficient time of the incubation of the thawed sperm samples. He states that if incubation period of the thawed samples is prolonged for 2h at 37°C, the sperm velocity results are getting more pronounced difference between the control and treatment groups, thus resulting in higher reliability and significance. This was previously pointed out in an article by Anel-Lopez, L. et al. in 2012 (31), where the treatment group samples supplemented with antioxidants did not significantly differ from the control group samples before they were incubated at 39°C. Ashrafi I. et al. in 2011, concludes that the semen quality and kinetic parameters are increased if the pre-thaw samples are being gradually equilibrated and diluted with the extender (33).
It can be concluded that this investigation did not find sufficient number of kinetic parameters to precisely determine the hypothesis that the presence of GSH in the soy-bean extender used after thawing of the samples, can increase their values, thus to improve the fertility capabilities of spermatozoa.
Two of the parameters that were marked with statistical difference (VSL and BCF) could not be precisely appointed as indicators for GSH effect if present in the semen extender, since only one ram ejaculate from the treatment group has been detected to influence variability of the results in regards of the BCF.
The limitations of the investigation is the absence of dose-response curve, relaying the hypothesis entirely on previous reports regarding the effective concentration (5).
Future investigations that will regard the report of the present article should use longer time of incubation (2 hours) for the thawed semen in order to acquire statistical significance between the groups for more kinetic parameters, at temperatures of 37°C or 39°C (16, 31). Semen handling protocols should include gradual equilibration and addition of extender in the thawed semen (33).
This study was intended as probing investigation for future implementation of assessment protocols of thawed ram semen and to possibly relate it with GSH as additive in the semen extender. We hope that results and conclusions can help not only the people that were involved, but other young researchers that have the will and potential of experimenting in ram semen cryopreservation which has proved to be a difficult topic for many scientists.