INTRODUCTION
Recurrent airway obstruction in horses (RAO), otherwise known as heaves, is one of the most common pathology of the respiratory system of horses. Its previous name Chronic Obstructive Pulmonary Disease (COPD) derived from human medicine, defines the symptoms characteristic for smokers and inaccurately refers to a disease occurring in horses. This is due to the pathophysiology of the disease and its different course. RAO is a disease that occurs in middle-aged horses, consisting of inflammation and gradual bronchospasm with bronchial walls rebuilding, and excessive production of mucus (1). It occurs most commonly in horses kept in stables, exposed to dust and fed low-quality hay. RAO occurs in horses aged seven years and over, and the incidence increases with age. Some authors considered the genetic background as a predisposing factor, which results from the fact that in some breeding lines RAO is frequently observed (2, 3).
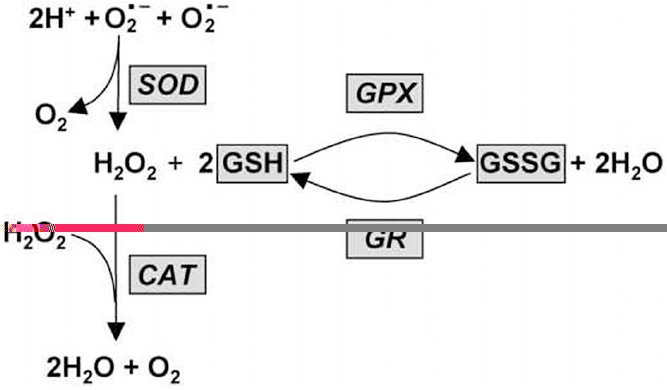
Aerobic organisms use oxygen for their metabolism, therefore they need to develop a number of mechanisms to prevent excessive formation of reactive oxygen species (ROS). Studies on free radicals began in 1954, when Commoner et al. (4) published a study on ROS. Another groundbreaking report, regarding the participation of free radicals in the aging process is authored by Harman (5). In thee sixties superoxide dismutase (SOD) was discovered, which is an enzyme neutralizing superoxide anion (O2·-), (6). Reactive oxygen species are involved in many cellular processes, but their excessive quantity leads to oxidative stress, which manifests itself by damage to proteins, nucleic acids and lipids. The role of antioxidants is played by fully specialized enzymes and low molecular weight non-enzymatic antioxidants. The body’s defense mechanisms against free radicals include: preventing the formation of ROS and their reactions with biologically important compounds (prevention), interrupting the free radical chain reaction and undesirable non-radical oxidation (termination and intervention) and removing the effects of ROS reactions with biomolecules (elimination or repair). The first line of defense is the so-called enzymatic triad (Fig. 1), i.e. superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) as well as the proteins binding the transition metals (7).
Figure 1. Diagrammatic representation of the relationship between antioxidant enzymes, GSH and GSSG. SOD, Superoxide dismutase; CAT, cat-alase; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione
SOD is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O2−) radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. CAT catalyzes the decomposition of hydrogen peroxide to water and oxygen (8). It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Likewise, catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert approximately 5 million molecules (9) of hydrogen peroxide to water and oxygen each second. GPx is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage (10). The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water. The second line of defense mainly involves low molecular weight antioxidants, or free radical scavengers. The third line of defense against oxidative stress is the ROS reaction with cellular macromolecules.
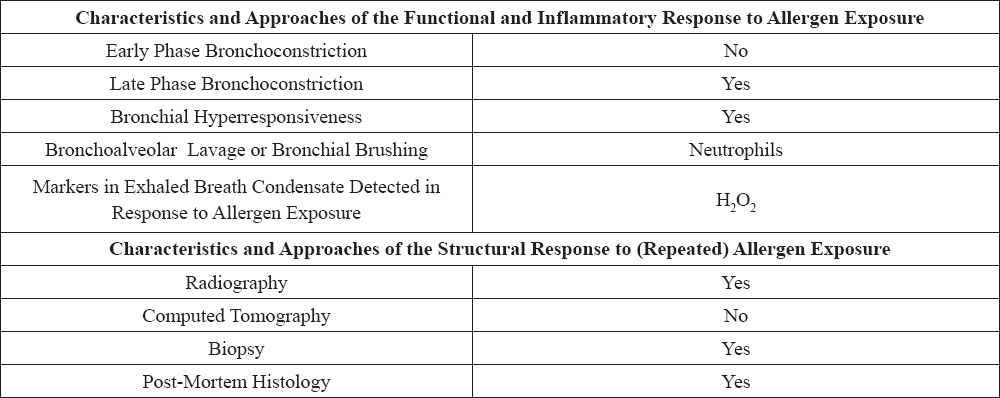
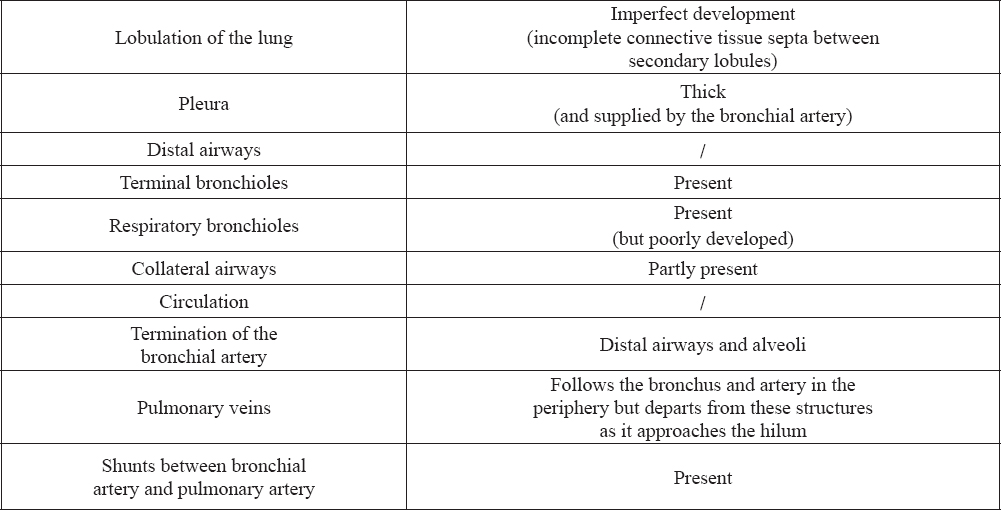
A significant contribution of reactive oxygen species to a number of lung diseases in humans has been confirmed (11), among others, to acute respiratory distress syndrome (12), asthma (13) and chronic bronchitis (14). Horses are one of the alternative models for human asthma (15) (Table 1, Table 2). Studies on the body’s response to the ROS in the case of human respiratory diseases are well known. Research on oxidative stress in horses with RAO has been conducted previously, although ambiguous results have been reported (16, 17). Moreover, most of the studies relate to the local effects of oxidative stress in the airways. Only a few studies investigated systemic markers of oxidative stress in RAO-affected horses. Results from these studies are difficult to compare because of differences in methodologies used and type of biomarker investigated. Despite the diversity of research models, namely exposing animals to allergens or factors having a negative impact on the body or subjecting them to stress tests, the studies did not allow for an in-depth analysis of the body’s response to the negative effects of oxidative stress in RAO (16, 17, 18).
Table 1. Horse as alternative model of human asthma
Table 2. Characteristic of horse lungs according to secondary lobulation
GLUTATHIONE LEVEL IN RAO CRISIS
Art et al. (19) noted that the exacerbation of RAO in horses caused significant changes in the lungs, including changes in the number of neutrophils in bronchoalveolar lavage (BAL). Research on reduced glutathione levels (GSH), glutathione disulfide (GSSG) and glutathione redox ratio (GRR) in the blood showed no significant differences between the group of healthy horses, horses with RAO in remission and those in acute phase of the disease. On the other hand, the values of those indicators measured in bronchoalveolar lavage fluid were significantly higher in horses with RAO in acute phase compared to healthy horses. There was also a significant correlation between the GRR, GSSG and neutrophil counts. These studies seem to confirm the hypothesis that oxidative stress is a secondary phenomenon to the increase in the number of neutrophils in the lower respiratory tract, which is associated with inflammation. Olszewski and Laber (20) confirmed the primary role of neutrophils, which are most stimulated among all the cells when animals are in acute phase. Art et al. (19) presented interesting relationship between the level of GSSG and GSH, because the increase in the level of GSSG should be followed by a decrease in the level of GSH. However, in their research the GSH level was high. This mechanism may be associated with increased release of the intracellular GSH type II pneumocytes due to increased membrane permeability, cell lysis or active transport (21).
PHYSICAL EFFORT AND THE ANTIOXIDANTS LEVEL
A research conducted by Kirschvink et al. (22) on the impact of chronic airway inflammation and physical effort on antioxidant status of horses with RAO showed both systemic and localized changes in the values of antioxidants. Difference of GSH, GSSG and GRR levels in blood of healthy horses and those with RAO, measured after physical effort was significant. The increase in these values suggests that systemic RAO glutathione synthesis is enhanced. The level of uric acid (UA) in horses with RAO after physical effort was significantly higher compared to healthy horses. The reduced ability to deliver oxygen during exercise increases oxygen deficit, which is associated with obtaining energy through anaerobic respiration. Its consequence is the accumulation of uric acid (23). Levels of markers of antioxidant status obtained from bronchoalveolar lavage were more diverse than those measured in the blood. This could be related to difficulties in standardizing the methods of collection. Horses with RAO in remission had similar values of GSH, GSSG and GRR and even exercise did not significantly change their values. These results are consistent with previous studies concerning dysregulation of GSH synthesis in the lung in horses with RAO (19), and people with asthma (24, 25). UA levels were higher at rest in horses in acute phase. UA 60 minutes after exercise was significantly higher in healthy horses and horses with RAO in remission. UA concentration was correlated with the percentage of neutrophils in BAL, which may suggest that airway inflammation leads to an increase in the local synthesis of uric acid (26).
EFFECT OF ANTIOXIDANT SUPPLEMENTATION
There are many studies indicating that the consumption of a diet rich in antioxidants may have a beneficial effect on chronic respiratory diseases such as asthma and COPD in humans (27, 28, 29, 30). These reports have been used by Kirschvink et al. (31), who aimed at determining the effect of oral supplementation of antioxidants on the levels of antioxidants in blood and bronchoalveolar lavage fluid in horses. The antioxidant supplement (AS) consisted of a mixture of natural antioxidants including vitamin E, vitamin C and selenium from a variety of sources (based on Winergy Ventilate Ventil-ate Respiratory Supplement (R) (UK)). The placebo consisted of oat feed pellets without additive. Either AS or placebo (250 g) were fed once daily with 1 kg wheat concentrate (molassed oats, pressed corn and pellets). The results indicated that the plasma level of UA was significantly lower after the supplementation than before antioxidant (P<0.02), and placebo (P<0.02) administration. The exercise caused a statistically significant (P<0.05) increase in the level of UA in bronchoalveolar lavage fluid in all groups. The increase in plasma levels of UA during and after exercise reflects the loss of adenosine-5’-triphosphate and purine metabolism in muscle, which produces hypoxanthine (32). During intense exercise, xanthine dehydrogenase is converted into xanthine oxidase. This enzyme catalyzes the conversion of hypoxanthine to uric acid and xanthine associated with the release of the superoxide anion. Thus lower level of uric acid is associated with a reduction in purine metabolism, reduction in ROS production and oxidative stress reduction. Mills et al. (33) demonstrated that administration of allopurinol which is an inhibitor of dehydrogenase and xanthine oxidase significantly reduces the synthesis of UA and GSSG in healthy horses during exercise. In people with COPD undergoing physical training, the administration of allopurinol reduced the level of GSSG and malondialdehyde (MDA) (34). Different levels of GSH, GSSG and TGSH obtained in these studies confirmed previous report that chronic inflammation of the respiratory system such as asthma in humans (24) and RAO in horses (19) dysregulates the metabolism of these compounds.
Youssef et al. (35) presented a study in which they evaluate the effect of a combination of sodium selenite and ascorbic acid on clinical outcome, antioxidant enzymes, and trace elements status in horses with lower airway disease. For this purpose, 40 draft horses with lower airway disease were randomly selected. For all groups, each horse was administered antibiotic, non-steroidal anti-inflammatory, and mucolytic drug. In addition, 2 groups (each ten horses) were injected with 15 mg/kg sodium selenite and 30 mg/kg ascorbic acid every 24 h for successive 4 weeks. The present result is supported by the suggestion of Kirschvink et al. (36) who reported that the beneficial effect of antioxidants supplementation on the oxidant/antioxidant equilibrium should only be expected after a few weeks of its supplementation. Regarding the antioxidant enzymes activity, GR activity was significantly decreased after sodium selenite and ascorbic acid supplementation. This result could be attributed to the increased activity of theglutathione antioxidant system as indicative of improved antioxidant potential with subsequent increase of the intracellular synthesis of glutathione. GST and CAT activities were increased after antioxidant supplementation. This act may be due to increase and modulation of cellular glutathione redox system due to sparing effect of vitamin E on glutathione utilization for the scavenging of lipid peroxidation reactions (36, 37, 38). In order to confirm the hypothesis that airways inflammation (39) and hyper-responsiveness observed in equine passively sensitized bronchi could be related to an increased production of ROS, Matera et al. evaluated the effects of a pretreatment with GSH on either non-sensitized or passively sensitized bronchi. They observed that a pretreatment with GSH significantly decreased the IL-1β mRNA expression and the contraction induced in passively sensitized tissues, but did not show any effect in the non-sensitized ones. In order to justify these different responses, we must consider that under normal circumstances ROS are kept under tight control by SOD enzymes (40). In acute and chronic inflammation, the production of ROS increases at a rate that overwhelms the capacity of the endogenous defense system to remove them (40). Moreover, GSH is an important component of the lung antioxidant defense (41). Therefore, a supplementation of this antioxidant agent is necessary to protect lungs from oxidative stress during acute or chronic inflammation. Based on this analysis, it can be confirmed that oral supplementation may have beneficial effects on antioxidant status of horses suffering from RAO.
Seeking an effective remedy for RAO, several agents have recently been tried. The study presented by Venugopal et al. (42) evaluated potential therapeutic agents, namely pentoxifylline, PDTC and endothelin A and B antagonists (in combination), for their effectiveness in reducing reactive species levels in pulmonary tissues. Being a methylxanthine derivative, pentoxifylline is a nonspecific phosphodiesterase inhibitor and has smooth muscle relaxing properties (43). Ammonium pyrrolidine-dithiocarbamate is an antagonist that inhibits activation of nuclear factor-kB, a regulator of transcription, which comes later in the inflammatory reactions (44). Upregulation of endothelin receptor function has been reported in airway inflammation (45, 46), and blockade of its receptors could have beneficial effects in RAO. As expected, all these agents were effective in reducing ROS in affected horses. However, only pentoxifylline and endothelin antagonists were effective in reducing RNS. The inability of PDTC to reduce peroxynitrite suggests that upstream inhibition of inflammatory changes is needed to control the progression of pulmonary inflammation. The present study agrees with previous reports that pentoxifylline given to RAO-affected horses improved their respiratory function (47), possibly by reducing reactive species stress.
EXPOSURE TO OZONE AND THE ANTIOXIDANTS LEVEL
Ascorbic acid (AA) is an important, non-enzymatic antioxidant. It reacts with inhaled oxidants, for example, ozone (48) as well as those of neutrophil origin, i.e., hydrogen peroxide or hypochlorous acid (49). Earlier reports indicate potentially beneficial role of ascorbic acid in the treatment of asthma and bronchospasm (50, 51). As one of the first Deaton et al. (52) studied the impact of ascorbic acid on the pathology of RAO in horses and inflammation on the body’s antioxidant levels. In these studies, horses with RAO had significantly lower levels of AA in both plasma and bronchoalveolar lavage compared to healthy horses. The results of the statistical analysis confirmed the relationship between the number of neutrophils in bronchoalveolar lavage fluid and the level of AA (P < 0.001) in the group of horses with RAO. Neutrophils may affect the level of AA by inducing oxidation that occurs as a result of ROS production (53).
Exposure to ozone causes a series of reactions in the human respiratory tract, including pulmonary function inhibition and causing airway inflammation (54). Ozone in the upper and lower respiratory tract reacts with unsaturated fatty acids, proteins, and antioxidants, such as ascorbic acid and reduced glutathione (55). The effects of ozone on the respiratory system’s response in the horse were researched by Deaton et al. (56). Their results indicated that horses with RAO that are not in the remission phase are not more sensitive to the compound’s activity compared with clinically healthy horses. The concentration of AA, amino acid redox ratio (AARR), UA, GSH, GSSG and GRR did not differ significantly between the group of healthy horses and those suffering from RAO before ozone exposure, and the concentration did not change after animal exposure to the compound. A significant part of the administered ozone reacts with a variety of compounds, most likely with fats and proteins (24, 55), which reduces its potentially harmful effects. It is possible that the doses and time points that authors selected were not optimal for assessing the effects of O3 on antioxidants (57, 58). RAO-affected horses in remission are not more sensitive to ozone despite a decreased pulmonary antioxidant capacity. Sensitivity to ozone appears to be independent of initial pulmonary antioxidant status.
EXPOSURE TO MOLDY HAY AND MYELOPEROXIDASE CONCENTRATION
Myeloperoxidase (MPO) is a haemoprotein stored within the granulocytes, catalyzing the formation of hypochlorous acid which has oxidizing properties. Its level in tissues and body fluids has been studied in humans and experimental animals. It can be used to determine the level of degranulation and activation of neutrophils (59, 60). Myeloperoxidase levels in bronchoalveolar lavage in horses were described as the first by Art et al. (61). In horses with RAO, exposure to moldy hay caused a significant increase in the number of neutrophils and MPO concentration in bronchoalveolar lavage fluid. After 2 months of grazing, lung function returned to physiological values, and the number of neutrophils and the concentration of MPO significantly decreased. Although there was no significant difference in the number of neutrophils in bronchoalveolar lavage fluid between a group of healthy horses and those with RAO during remission, it was noted that the level of MPO in horses with RAO during remission was significantly higher than in the clinically healthy horses. This relationship shows that inflammatory cells, neutrophils in this case, are able to maintain an increased level of activation for a long time (at least two months) after exposure to environmental irritants (61).
THE ENZYME TRIAD IN RAO
There are very few publications found in the scientific literature related to RAO in horses about the most important line of defence against free radicals, namely the enzyme triad. Their most important function is to prevent the formation of ROS and their reactions with biologically important compounds. Antioxidant enzymes in horse pathology have been described, among others, in the course of oxidative stress following (62, 63). Authors compare enzyme activity in a group of horses with RAO and those clinically healthy (64). The results of these studies showed a significantly impaired activity of antioxidant enzymes in horses with clinical form of RAO. This condition indicates intensified formation of free radicals and their active participation in the pathogenesis of recurrent airway obstruction. Some studies showed a statistically significant increase in the activity of antioxidant enzymes in ill horses, while other studies showed a decrease in activity of these enzymes (18, 22). Inconsistent results may be associated with the exacerbation of symptoms, horse maintenance or any treatment administered (1). High activity of antioxidant enzymes in different studies and low in others suggested that redox system is upregulated during exposure to dusty straw and hay to combat oxidative stress (22, 65). Ramery et al. (66) proposed distinct mechanisms to explain local and systemic oxidative stress related to airway inflammation. Local oxidative stress occurring in the lung is induced by inflammatory cytokines such as TNF and IL1, and by respiratory burst by activated macrophages and neutrophils (67). The mechanism proposed for systemic oxidative stress involves intermittent hypoxia which activates systemic NOX and other ROS-producing enzymes (68). Whether changes of GPx activity in peripheral whole blood have potential in diagnosis and monitoring of IAD in horses needs to be investigated in future studies evaluating sensitivity and specificity, and predictive value.
CONCLUSION
Recurrent airway obstruction (RAO) is currently one of the most frequently encountered pathologies in the horse. Production of reactive oxygen species is a secondary phenomenon to the disease. Numerous studies point to the deregulation of the activity and synthesis of antioxidants in the course of RAO. Chronic inflammation intensifies ROS generation, which determines oxidative stress and exhaustion of reserves of antioxidants in the body. The severity of symptoms, animal maintenance and pharmacological therapy are associated with the level of free radical formation. The use of oral supplementation with antioxidants appears to be justified in order to maintain the oxidative and antioxidative balance. Studies are being conducted now to better define the role of antioxidant enzymes in the course of RAO in horses.