INTRODUCTION
Fluoroquinolones are a group of synthetic antimicrobial agents widely used in human and veterinary medicine for treatment and prevention of infections caused by Gram-negative and Gram-positive bacteria (1). They are among the most often prescribed antibiotics in fisheries (2), but their use in aquaculture presents a risk to fish products’ consumers, because of potential risk of residues in the food. Tissue disposition of fluoroquinolones, including enrofloxacin and ciprofloxacin, in fish species depends on several factors such as ambient water temperature and salinity, lipid content and properties of the drug molecule (3, 4). These factors have significant impact on the withdrawal time and depletion of residues from muscles and can be a prerequisite for the presence of undesirable levels of drugs. Information about the behavior of residues of antibiotics during frozen storage is scarce. Alfredson and Ohlsson (5) found significant reduction of sulfamethazine residues in muscle kept for 3 months at a temperature of -20 °C. The average decrease was 35% in bovine and 55% in porcine muscle (5). Other data showed a significant decrease of ampicillin residues in pork meat during the first 3 months of storage at -20 °C (6). Residual concentrations of ampicillin were found in the pork muscles after 8 months of storage at the same temperature (6). In our previous studies major reduction of the residues of amoxicillin (7), kanamycin (8), tobramycin (9), and ampicillin (10) in poultry or rabbit meat during storage at -18 °C was found. No significant reduction of trimethoprim in poultry meat (8) and gentamicin in rabbit meat was registered (10). In all cases, microbiological methods were used which limited the ability for detection of metabolites. This data shows that stability of antibacterial substances in mammals’ and chickens’ muscles differs significantly, depending on the individual drug and animal species. Moreover, little is known of the stability of antimicrobial drugs in fish muscle after different storage times at usual commercial conditions.
Because of insufficient data concerning the changes of antimicrobial residues in meat during storage, the aim of the present study was to investigate concentration changes in enrofloxacin and its metabolite ciprofloxacin contents in trout and carp meat during frozen storage.
MATERIAL AND METHODS
Drugs
Enrofloxacin (Baytril 5%, Part. No KP076SM, Bayer Animal Health Gmbh, Leverkuzen, Germany) was used for oral (p.o.) treatment of fish as 1% solution. Analytical standards of enrofloxacin hydrochloride (Part No. 20020323) and ciprofloxacin hydrochloride (Part No. FPCPF 070483) were supplied by Biovet Pestera (Bulgaria) and Actavis (Bulgaria), respectively.
Animals
Two years old rainbow trouts (Oncorhynchus mykiss, n=20) with mean body weight of 450 g were included in the experiments. They were supplied by a fish farm, located in Enina (Bulgaria). The animals were placed in two aerated tanks. Water temperature was 15°C and pH was 7.5.
Market-sized carps (Cyprinus carpio, n=11) with a mean body weight of 1000 g were purchased from a commercial fish farm in Nikolaevo (Bulgaria). The fish were placed in 2 tanks, containing 800 l of tap water (water temperature 17°C; pH 7.2) with constant aeration.
Experimental procedure
The experimental design was approved by the Ethics and Animal Welfare Committee at Trakia University, Stara Zagora. Euthanasia was performed under the Directive 2010/63/EU on the protection of animals used for scientific purposes requirements.
Control groups of trouts (n=5) and carps (n=5) were not treated with enrofloxacin and were used for obtaining of muscle tissue samples for preparation of blank samples and fortified samples with standard dilutions for quantitative analysis of the drugs.
The other trouts (n=15) and carps (n=6) were treated orally with a single dose of enrofloxacin via a gavage tube (1% solution at dose rate of 10 mg/kg body weight). Tissue samples were obtained from each fish after euthanasia by a percussive (manual) blow on the cranium with a hammer. Muscle samples were taken from six fishes from each species 24 hours after the treatment with the antibacterial agent. Each muscle sample was divided into six portions and they were stored at -18 °C. These samples were analyzed at day 0 (immediately after obtaining the samples), 15, 30, 90, 180 and 270 of the storage. The remaining 9 trouts were euthanized and stored as whole fishes (with internal organs), without cleaning and muscle samples were analyzed 180 and 270 days after the storage. Aqueous solutions of enrofloxacin hydrochloride and ciprofloxacin hydrochloride (1 mg/mL) were stored at the same conditions as samples for depletion check of the pure standards. These solutions were analyzed together with the muscle samples at the same time intervals.
HPLC analysis of enrofloxacin and ciprofloxacin concentrations
The tissue samples from muscles (0.5 g) were homogenized in 0.5 mL of 0.1 M phosphate buffer pH 7.4 with a homogenizer at high speed up to 1 min. To this homogenate, 6 mL of dichloromethane were added, and the samples were mixed for 1 min and centrifuged for 6 min at 1000xg. The aqueous layer was removed. The organic phase was collected in 10 ml tubes. It was evaporated in a vacuum evaporator (CentriVap Vacuum Concentration System, Labconco, Kansas, MO, USA) at 40 °C to dryness. The residue was dissolved in 500 μL of demineralized water. Aliquots from 20 µL were injected into the HPLC system.
HPLC system was equipped with Hypersil Spherisorb ODS-2 (C18)-150 4.6 -mm 5 μM column, a Surveyor LC Pump Plus, a Surveyor fluorescence detector, and a Surveyor Autosampler Plus (Thermo Fisher Scientific Inc., USA). The column was used at room temperature. The mobile phase consisted of acetonitrile in aqueous solution (25:75, v/v) of potassium dihydrogen phosphate (0.05 M) in water. The pH of the mobile phase was adjusted to 3.5 with phosphoric acid (85%). The flow rate was 0.6 mL/min, isocratic conditions. Excitation and emission wavelengths were set at 277 nm and 418 nm, respectively for determination of enrofloxacin and ciprofloxacin. Peak area integrations were carried-out using ChromQuest Chromatography Data System (Thermo Fisher Scientific Inc., USA). Standard curves for both fluororquinolones were prepared with tissues from untreated animals. The concentrations of calibration standards for enrofloxacin and ciprofloxacin were 1000, 750, 500, 250, 100, 50, and 10 ng/mL. Retention times for enrofloxacin and ciprofloxacin were 3.88 min and 4.57 min, respectively. Limit of detection and limit of quantification for both compounds were 10 and 50 ng/mL. The overall recovery rate in tissue samples exceeded >75%.
The analysis was performed in the Central Scientific Laboratory of Trakia University.
Statistical analysis
Descriptive statistics was performed with Statistica for Windows (Statistica 6.0.1, USA). Data were presented as mean ± SD. Statistical analysis was performed with Mann–Whitney test. – P level < 0.05 was considered as significant.
RESULTS
Rainbow trout (Oncorhynhus mykiss)
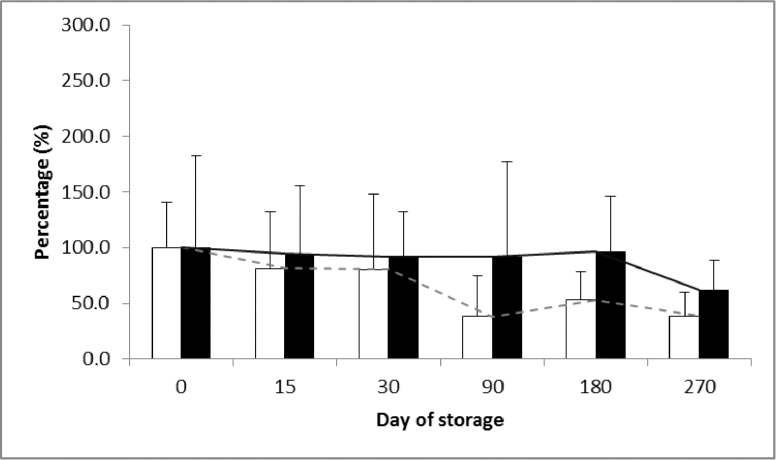
Enrofloxacin residues in the meat of rainbow trout showed insignificant decrease during frozen storage the first six months (Table 1). Considerable decrease was observed after storage during the last 3 months, after 270 days of storage. The levels reached 3.69 µg/g, or 62% of the initial values (Fig. 1).
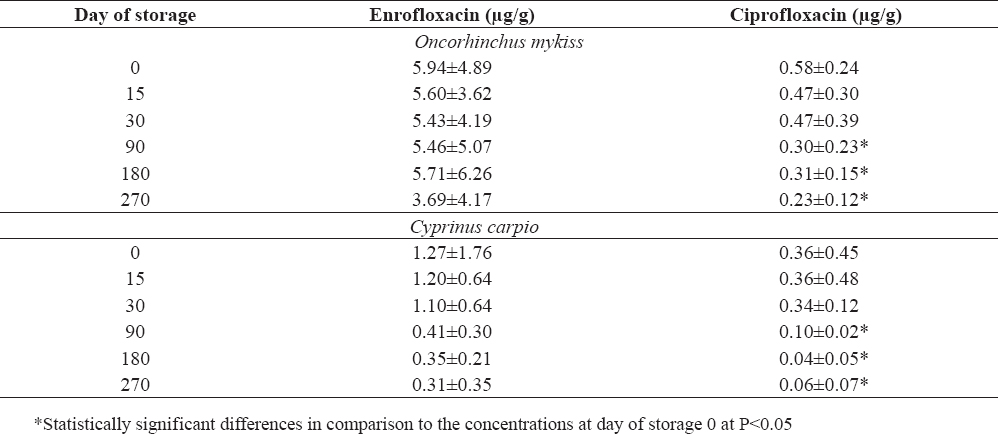
Table 1. Residues of enrofloxacin and ciprofloxacin in muscle samples (Mean ± SD) from trout (Oncorhinchus mykiss) and carp (Cyprinus carpio) after storage at -18 °C
Figure 1. Levels of residues of enrofloxacin and ciprofloxacin, presented as percentage of the day 0 of storage, in muscle samples from trout (Oncorhinchus mykiss) after storage at -18 °C. and gray dashed line represent data for ciprofloxacin; and black line represent data for enrofloxacin
Ciprofloxacin, which initial concentration (at 0 day) in the trout meat constituted about 10% of the enrofloxacin level, showed tendency for a gradual decrease. During the first 2 weeks of storage a decline of approximately 19% was observed (P>0.05) and subsequently after 9 months of storage, the ciprofloxacin concentration in the meat was less than 50% of the initial concentration (P<0.05). Significantly lower ciprofloxacin levels were measured after 3 months of storage (P<0.05).
The fish, stored without being sliced and eviscerated, showed different tissue levels of both fluoroquinolones as compared to the muscle samples which were cut into small pieces before storage. After 9 months of storage the enrofloxacin and ciprofloxacin residues were 8.24±3.99 µg/g and 0.56±0.47 µg/g, respectively. These values were higher than those obtained from the precut muscle samples (P > 0.05). Enrofloxacin concentrations were 38 % higher and the amount of ciprofloxacin was similar if compared to the initial concentrations of the drugs in the muscles.
Common carp (Cyprinus carpio)
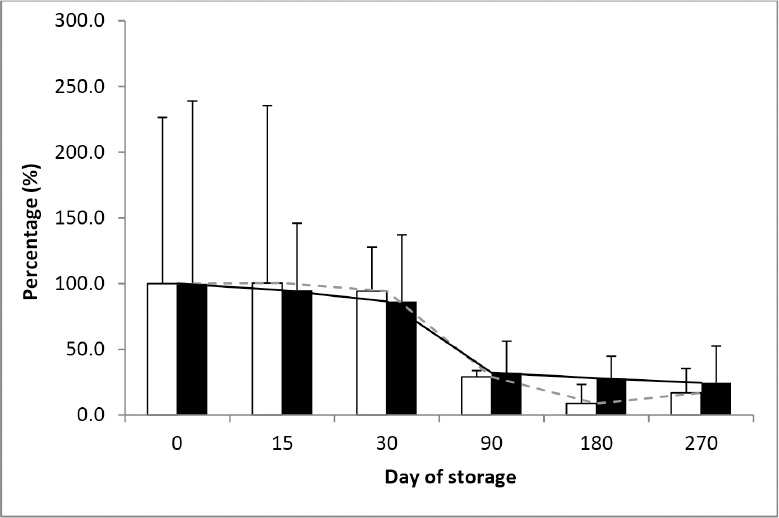
Results obtained from the analyzed carp muscles showed that during the first month of storage there were no statistically significant changes in the tissue levels of both quinolones (Table 1). After 90 days of storage at -18 °C, enrofloxacin concentration decreased to 30% of the initial level and remained almost unchanged up to the end of the study (Fig. 2). Ciprofloxacin level continued to drop even after 90 days of storage and at the end of the storage period its concentration constituted approximately 1/6th of the initial values. Its concentrations were statistically significantly lower after 3, 6 and 9 months of storage (P<0.05).
Figure 2. Levels of residues of enrofloxacin and ciprofloxacin in muscle samples from carp (Cyprinus carpio) after storage at -18 °C. Data are presented as percentage from the day 0 of storage. and dashed gray line represent data for ciprofloxacin; and black line represent data for enrofloxacin
DISCUSSION
Rearing of fish species in farm conditions and international trade with aquaculture products have significantly developed during the last years. These tendencies determine extensive use of antibacterial drugs to control increased rates of diseases. Enrofloxacin is among the few drugs licensed for use in aquatic species with established Maximum Residual Limits of 100 μg/kg as the sum of the levels of enrofloxacin and ciprofloxacin in tissues (11). Literature data indicates variable withdrawal time in different fish species (12). Therefore depletion of drugs from edible tissues must be assessed in order to offer safe food on the market. In fish species, additional obstruction is the dependence of residues depletion time on the water temperature (4). Although they adopted strict criteria, there are cases of illegal use of drugs without following the required withdrawal time and Maximum Residual Limits which can be a reason for existence of residues in processed fish and requires information about the stability of enrofloxacin and its active metabolite under conditions of storage.
The data from our study show differences between trout and carp in the depletion time of fluoroquinolones after storage of muscle samples at -18 °C. Storage of trout meat for six months resulted in absence of significant changes (P>0.05) of enrofloxacin residues in meat and the highest decrease was observed after nine months. Insignificant changes were found in both enrofloxacin and ciprofloxacin levels in carp muscle during the first month of storage. Significant decrease of enrofloxacin levels was observed 90 days after freezing, which was earlier in carp samples if compared to trout. Similarly to these results, the concentrations of enrofloxacin in water samples frozen at -20 °C for 28 days, were stored up to 90% in comparison to the samples, analyzed immediately (13). After 6 months of preservation of stock solutions of enrofloxacin at -20 °C the loss was less than 10% than in Rainbow trout fish meat, kept in the same freezer in the current study. After a longer period of storage, concentrations of fluoroquinolones in frozen water samples dropped off dramatically as in our experiment (14). Similar data were reported for tetracyclines, aminopenicillines and sulfonamides in frozen water in the EPA study (13) and in frozen meat samples. Verdon et al. (6) found 40% lower concentrations of ampicillin in pork meat kept at -20°C for 235 days and absence of changes at -70 °C. Trimetoprim degraded up to 50% in chicken meat after freezing at -18 °C for 30 to 90 days (8). Gentamicin was stable in rabbit’s meat stored at same conditions for 45 days (10).
Enrofloxacin is dealkylated to pharmacologically active metabolite ciprofloxacin in several fish species, including trout and carp (15, 16, 17). Ciprofloxacin was found in the muscles of both investigated fish species and its depletion from this tissue differs from the data for the parent compound. Results showed that 15 days of storage lead to reduction of ciprofloxacin concentrations by 20% and by more than 50 % after the third month till the end of the experiment in trout muscles. The observed higher concentrations of enrofloxacin and ciprofloxacin in samples from whole trout, stored with internal organs, can be explained by additional penetration of fluoroquinolones to the muscles. Depletion pattern of ciprofloxacin concentrations in the carp muscles was similar to those in the samples from trout. Similarly, another fluoroquinolone compound such as gatifloxacin was stable in human plasma for 7 weeks at -20 °C (18).
CONCLUSION
Our data indicates that the samples from rainbow trout and their products can be stored frozen at temperature under -18 °C for a period of 6 months and to be analyzed within this term for determination of residues from enrofloxacin. For common carp meat, this period is 3 months. Administration of the drugs in modern aquaculture including antibiotics, requires knowledge for the withdrawal time of the drugs in each fish species. Freezing for months of whole and eviscerated fish results in different levels of fluoroquinolones in the muscles. Less residues are found in eviscerated fish during storage at - 15, 16, 17 °C. A strong tendency toward enrofloxacin and ciprofloxacin decrease was observed in trout and carp muscles, although these drugs can be found in the samples after months of storage.