INTRODUCTION
Ticks are obligate haematophagous ectoparasites which have multiple adverse effects on the host organism. A particular problem is that they spread diseases to humans, domestic and wild animals, which can be reservoirs, vectors and/or transient hosts for the tick-borne pathogens (5, 12, 14, 20, 23, 35, 36, 37, 41).
Most of the tick species of the family Ixodidae are egsophilic and during the search for the host they inhabit open habitats. During this development, ticks go through four life stages. These stages are egg, larvae (or seed tick), nymph, and adult (1, 5). Ixodidae ticks mainly belong to three host life cycle group of ticks, while during each developmental stage it feeds once on a separate host and will remain attached to the host for several days (8, 43).
An important feature of the life cycle of ticks is the diapause (10, 15). Throughout diapause, a tick goes into hibernation, during which the metabolism slows (49, 50). Diapause allows ticks to survive adverse conditions, such as extreme temperatures, drought and lack of food (19).
We have taken into account the epidemiological significance of ticks, because they carry many disease pathogens that belong to the group of natural focal infections, characterized by endemic and seasonal occurrence (27, 41). That was the starting point of our investigation of domestic animals tick fauna in certain areas of Serbia. In our paper we present the tick fauna of sheep and goats from the area of Šabac (Serbia) in the 2010-2012 period.
MATERIAL AND METHODS
Sheep and goat breeds play important role in the Šabac area. Usually, they are kept in small herds of 20 to 25 animals. From early spring to late autumn, they are kept on pastures and graze on any land that is not being cultivated. It is common that flocks of goats and sheep are found on the same pastures. During the winter, goats and sheep are kept in stables. During the course of our study, we collected ticks from 52 flocks of sheep and 38 goat flocks. Ticks were collected monthly during the period from the exit to the pasture to the retreat to the barn (March to October). All specimens were placed into glass specimen bottles which had a piece of hard paper inserted bearing the name of locality, name of host and date and hour of collection. The tick species and sex/gender were identified by morphometric characteristics (24, 25, 47).
The data obtained wase analyzed using Chi-square test (χ2) to determinate if the tick species and the prevalence of infection with ticks depended on the host animal species. For all analyses, the confidence level was kept at 95%.
Our studies were carried out in the period from March 2010 to November 2012 in the municipality of Šabac, which is located at 44º 46‘ northern latitude and 19º 41’ east longitude and is in the western part of Serbia. Morphologically, the municipality of Šabac has three natural zones. North of the town there is a vast plain area known as Mačva where lowland humus is the dominant soil type. The second morphological unit constitutes the western part of the territory of the municipality of Šabac, which is characterized by a hilly relief - the Pocerina area, where the plain area Mačva gradually turns into a hilly area down to the Cer Mountain, where the relief and forest caused degradation and evolution of lowland soils into brown forest soil. The third morphological unit covers the southeastern part and it represents Posavina - the river valley along the right bank of the Sava River water intersected by former backwaters. There the most common type of soil is sandy black soil, which appears as a young Pedogenic creation and is most frequently found in the bottomlands of major rivers (2).
The municipality of Šabac has a continental climate. On the north side, Šabac is widely open to the Srem and therefore in terms of climate is strongly influenced by the Panonian continental climate. This climate is characterized by cold winters and warm, dry summers. The highest air pressure occurs in the coldest month of January, while the lowest in April. In this area winds blow from all quadrants. On windless periods as waste 1/3 the frequency of occurrence of winds. The mean air temperature in Šabac is around 11,7°C. Humidity is on average 78-92% in winter and 51-63% in summer. The annual precipitation is about 435 l/m2. Insolation increases from January to July and then decreases until December (official data by the Hydrometeorological Service of Serbia).
The climatic vegetation zones in this area correspond to the vegetation that is usually found on the territory of Serbia, such as forests of Hungarian oak (Quercus confestim), oak oak (Quercus) and beech (Fagetum montanuum), while in the underwater terrain we commonly encounter poplar (Populus alba) and white willow (Salicerum alba). With regards to bushes and low trees the most common are: mandrel (Prunus spinosa), hawthorn (Scataegus monogyna), wild service tree (Sorbus aria) and maple (Acer compestere). From the terrestrial plants, most common are: meadow fescue (Festuca pratensis), meadow grass (Poa pratensis), couch grass (Agropyrum repens), couch grass without awns (Bromus inermis), sheep fescue (Festuca ovina), French ryegrass (Arrhenatherum elatius), red clover (Trifolium pratense), common vetch (Vicia sativa) and meadow pea (Lathyrus sativa) (21, 22).
RESULTS
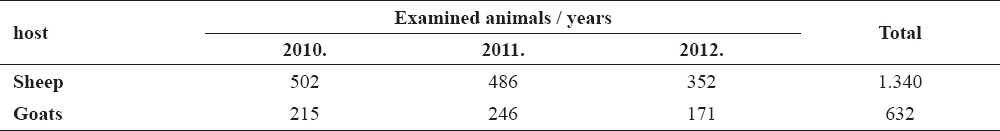
During our examination we examined 1.340 sheep and 632 goats (Table 1).
Table 1. Number of examined sheep and goats in the period 2010-2012
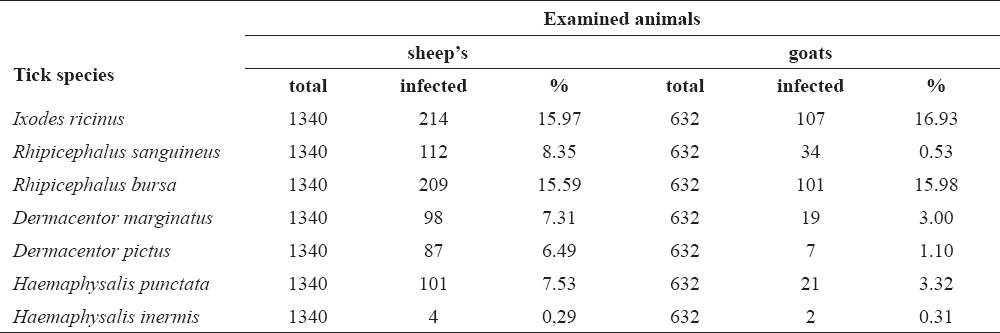
Tick infestation has occured in 15.97% (214/1340) of sheep and 16.93% (107/632) of goats. The results showed presence of Ixodes ricinus, Rhipicephalus sanguineus, R. bursa, Dermacentor marginatus, D. pictus, Haemaphysalis punctata and Ha. inermis. Their prevalence is presented in Table 2.
Table 2. Percent of sheep and goats infected with ticks in the period 2010-2012
Relative abundance analysis revealed that the I. ricinus was absolutely dominant species in sheep, followed by Rhipicephalus bursa, R. sanguineus, Haemaphysalis punctata, Dermacentor marginatusand D. pictus and Ha. inermis. Similar fauna of ticks was found in goats, since the herds of goats graze at the same pasture areas with herds of sheep.
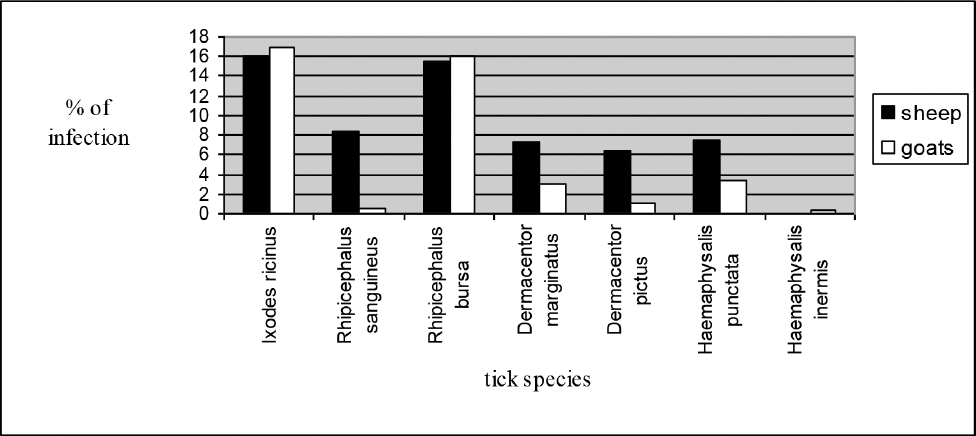
The chi-square test showed there was a diference present between sheep and goats with regards to the prevalence and intensity of infection with R. sanguineus, Ha. punctata, D. marginatus and D. pictus (Fig. 1).
Figure 1. Percent of sheep and goats infected with ticks in the period 2010-2012
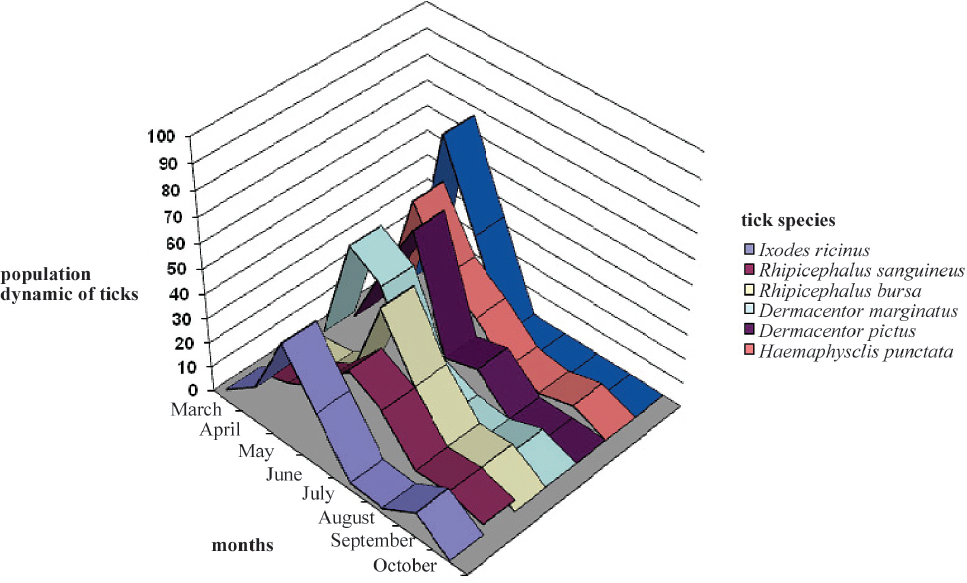
Figure 2. Population dynamics of the established species of ticks by months in the period 2010-2012
Population dynamics of the established species of ticks can be observed every year from March to October. No sampling took place from November to February, therefore their presence in nature cannot be confirmed. During the three years of examination, in the month of March the following types of ticks were found: Ixodes ricinus, Rhipicephalus sanguineus, Dermacentor marginatus and Haemaphysalis punctata. In April, we observed the occurrence of the following species: Dermacentor pictus, Rhipicephalus bursa and Haemaphysalis inermis. In April, species that reached maximum numbers were Dermacentor marginatus, Haemaphysalis punctata and Haemaphysalis inermis. Species Ixodes ricinus reached maximum abundance in May, in which we also found the maximum occurrence of the species Dermacentor pictus. In June, the population peak is observed for the species Rhipicephalus sanguineus and Rhipicephalus bursa, which are the most common types both in July and August. In September, we saw an increase in the population of two species of ticks: Ixodes ricinus and Dermacentor marginatus, while in October we observed the emergence of the following species: Haemaphysalis punctata and Ha. Inermis, and Rhipicephalus sanguineus and R. bursa.
According to the Chi-square test for averages of the three year sampling periods, there is no significant difference between population dynamics of tick species infection with sheep and goats.
Overall, the female-male ratio during the course of our study was 61.02% : 38.98% in favor of females, with a higher percentage of females established in all three years of research. The ratio of males and females of the same species is also interesting. Of the seven species established, a greater number of males than females (65.08% : 34.92%) occurred only in the species Rhipicephalus bursa, while for the other six species we established a larger number of females. For the two most commonly found species Rhipicephalus sanguineus and Ixodes ricinus, this ratio was 69.50%: 30.50% and 63.42%:36.58%, respectively in favor of females.
DISCUSSION
Comparison of the obtained results with findings in other regions of Serbia indicated that there is a great similarity in the established tick species. Examination performed in the Belgrade area established I. ricinus. R. sanguineus, D. pictus and D. marginatus as the most abundant species (12, 31, 32, 40, 44).
Results obtained during the examination of the tick fauna of sheep and goats in the northeastern, eastern and western part of Serbia showed that Ixodes ricinus and Dermacentor marginatus are the dominant tick species in those areas (31, 34, 35, 44, 46). Examination performed in sheep in the Prizren district (Kosovo) during 1991 (39) pointed to the presence of the same tick species, including Hy. savignyi, Ha. inermis, Boophilus calcaratus and Ornythonisus lachorenis. The latest examination conducted during 2013 in the Kumanovo area (Macedonia) established that most abudant in sheep were Ixodes ricinus, followed by Dermacentor marginatus, Rhipicephalus sanguineus, R. bursa, Haemaphysalis punctata and D. pictus (45). During all the examinations, Ixodes ricinus was the most abundant tick species insheep and goats. The found species of ticks are most common in sheep and goats in the regions of the Western Balkans, Mediterranean and Central Europe (14, 16, 25, 27, 28, 37, 38, 48).
Climate conditions have a great influence on the population dynamics of ticks. Population dynamics of ticks is related to the impact of climate factors like air temperature, relative humidity and rainfall (32, 49).
Our results confirmed the results of the studies carried out in northeast, eastern and south-eastern Serbia (29, 33, 34) which established that I. ricinus was by far the most abundant species with the largest number of specimens collected in the spring at a temperature of about 15°C, relative humidity of 76.00% and rainfall of 81.11 l/m2. March marks the start of, the increase in the number of Haemaphysalis (punctata and inermis) species, whilesimilar results were obtained during examination in France (26), Romania (28) and in Macedonia (45). April is the month of greatest abundance of D. marginatus, Haemaphysalis punctata and Ha. inermis which reached their peak at a temperature of 9.01° C, relative humidity of 75.66% and rainfall of 35.80 and 36.06 mm/m2.
This data is in correlation with the results of other examinations of the seasonal dynamics of ticks in Europe (3, 6, 9, 11, 17, 29, 37). Similar values were obtained during research in Serbia (12, 41), France (26), Italy (27, 38) and in a Berlin forest (11). The authors point out that the low temperature, high humidity and rainfall significantly affect the life cycle of ticks, particularly the I. ricinus species (20, 28, 46). Same results were obtained worldwide e.g. in coastal areas of New York (7) and in South Africa (18, 20). Both studied the temperature below which the activity of ticks is completely stopped and the temperature at which it expressed its full activity and found a significant relationship of tick activities and the degree of reduction of temperature for adult forms. This agrees with the data published about ticks in various part of Russia, Central Europe and the West Balkans (4, 12, 13, 14, 20, 21, 25, 26, 29, 31).
The female abundance of established tick species has been in correlation with previously established population dynamics. The females of Ixodes ricinus species were present from March to October, with a peak population in May and June. Females of two species of the genus Rhipicephalus (sanguineus and bursa) have been found most often in the summer months - June and July. Findings of the females of species Dermacentor marginatus and Haemaphysalis punctata were most common in April and May, while sporadic finding of females of Dermacentor pictus and Haemaphysalis inermisspecies was attached to the spring months. This population dynamics of female ticks is characteristic for this microclimate (3, 8, 9, 15).
Males of the species Ixodes ricinus were found from March to October, with the spring peak population in May and autumnal in September which corresponds to the values obtained in our earlier research (12, 17). Males of the species Rhipicephalus sanguineus were established from March to October, a species Rhipicephalus bursa from April to September with a population peak of both species in June, which also corresponds to values for this geographical area (28, 30, 32, 38). Males of the two species of the genus Dermacentor (marginatus and pictus) were usually found from April to June, while the males of Haemaphysalis punctata species were established from April to June. A small number of males of Haemaphysalis inermis was found only in April which corresponds to values of research in this area and in Central Europe and the Mediterranean basin (16, 20, 35, 42, 47).
CONCLUSION
Being the vectors and reservoirs for many endemic tickborne pathogens, the tick transmit diseases that cause health disturbance in domestic animals and humans in affected areas.
The occurence of tick born diseases in this species is usual during the exposure time period. The population dynamics and the climatic factors that influence the tick population need to be studied in order to predict the critical points and implement adequate protection measures in animals with the final goal of disease prevention and control.