INTRODUCTION
At present, poultry has a big part in food resources and its production is higher than the large animal production in the world. In Turkey, 270 million poultry is produced each year and about 755.000 of these are geese. In regard to poultry production, Kars Province (East Anatolia Region) accommodates 0.36% of the poultry population in Turkey, but, Kars is in first place in Turkey, if considering the goose population, with 22% (1).
Geese can be raised in many different conditions in variety of climates. Geese production plays a very important role in the economy of Kars Province in Turkey and they have been popular in the region over the years. Geese are farmed in small flocks of 20-30 by the local people and these numbers can go up to 60-100 in a flock in the villages (2). Many families living in the city center and the rural area of the Kars Province fulfill their meat requirements by consuming a large amount of goose meat in their diet in winter (2, 3, 45) and a goose breeding station has been established in the region (4). The consumption of goose meat is rather distinctive and traditional in the region and shows dissimilarity to the rest of Turkey. The geese are raised organically in the fields and slaughtered at the beginning of winter, especially after the fall of the first snow, by individual families. After slaughtering, internal organs are eviscerated, slightly salted and hung to dry outside in the open air by the local people at home. Therefore, during the drying of geese carcasses outside in an open environment, they are exposed to dust and wind. Subsequently, dried geese carcasses are cursory stored to be consumed during the winter. Slaughtering of geese at home rather than at the poultry slaughterhouse is likely to be unhygienic, if care is not taken. Due to improper slaughtering conditions, processing usually cannot be considered as hygienic and furthermore, carcasses may be contaminated with microorganisms during the process (5).
Food intoxication is still a serious health problem in the country. According to recent reports, annually in the United Kingdom 17 million (31.6%) in Germany 23 million (28.7%), in USA 76 million (27.9%), in Turkey 19 million (27.8%) and in France 17 million (25.4%) of people are reported to be associated with food borne intoxication (6). The report of the Centre for Diseases Control and Prevention (CDC), based on data from the USA, revealed a grand total of 18.499 laboratory-confirmed cases of nine food-borne illnesses in 2008. Out of these laboratory-confirmed cases, the majority of food-borne illnesses were associated with Salmonellae (7.444 cases), Campylobacter (5.825 cases), Shigella (3.029 cases), Cryptosporidium (1.036 cases), E. coli 0157 (718 cases), Yersinia (164 cases), Listeria (135 cases), and Vibrio (131 cases) (6). Poultry meat is at the forefront of food intoxications. For example, between 1998-2008, the CDC received reports of 13.405 foodborne-disease outbreaks, resulting in 273.120 illnesses, 9.109 hospitalizations, and 200 deaths. The commodities implicated most commonly in the outbreaks were poultry (10%), beef (6.6%) and fish (6.1%) (7, 8). A recent report, by the Food Standards Agency, stated each year around 500.000 food poisoning instances, of which 244.000 could be attributed to poultry meat (6).
Microbiological studies on chicken meat and carcasses are well documented, but very few studies exist on goose meat and carcasses. The aerobic mesophilic bacteria, coagulase positive staphylococcus, Escherichia coli, Clostridium perfringens, listeria, Yersinia spp. and Salmonellae in fresh goose carcasses are investigated (9). Aydin et al. (10) showed that Campylobacter jejuni were common in the intestinal tracts of domestic geese and therefore geese have been considered as a potential reservoir for human and animal campylobacteriosis. However, these were alive birds. Turcsan et al. (11) examined goose liver samples in terms of anaerobic bacteria and clostridia, and enumerated Clostridium perfringens spores in the raw goose liver samples taken after evisceration of the birds (EB) in the slaughterhouse and after removal of blood vessels from the liver (RBVL) in the cannery. Because poultry feces contains Clostridium perfringens, the meat and viscera might be contaminated during processing (12). The samples taken after RBVL had significantly higher (p<0.05) spore counts than did those taken after EB, indicating contamination of the livers during the processing. The chemical and microbiological quality of fresh goose meat is investigated (13). However, recently it was reported very high (60%) prevalence for Salmonellae in carcass in examined flocks of domestic geese (14). Furthermore, high numbers of E. coli bacteria in the fresh goose meat are also reported (9). Thus, it is likely that dried geese carcasses can also be contaminated by pathogen micro-organisms. In this study therefore, dried goose carcass samples were collected from the local households in Kars/Turkey and microbiologically examined in terms of public health risks.
MATERIAL AND METHODS
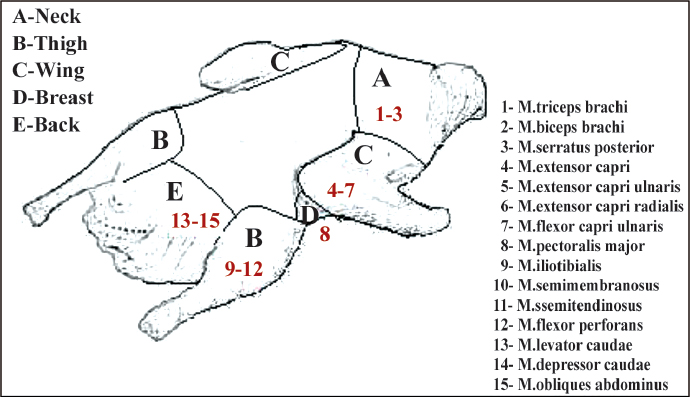
The dried goose carcasses were collected from the local shops and individual families. The carcasses were sampled from five different sites of each bird by excising an area of tissue as follows: 10 from neck, 12 from thigh, 10 from breast, 12 from wing and 12 samples from back of the birds. Thus, a total of 56 samples were investigated for their microbiological qualities in this study. The location of samples on the goose carcass is shown in Figure 1.
Figure 1. The location of samples on the goose carcass and muscles of parts
All the samples were homogenized in sterile porcelain cups. Starting with the first suspension of 10 g sample in 90 ml diluent, tenfold serial dilutions were made. Two 0.05 ml of each of the serially diluted samples were inoculated onto Plate Count Agar (Oxoid CM325) (15) for aerobic mesophilic bacteria, using the drop plating technique (double drops resulting in 0.1 ml per sample solution) (16). The plates were inoculated at 30ºC for 48-72 h under aerobic conditions. For the Enterobacteriaceae, Violet Red Bile Glucose Agar (Oxoid CM 485) was inoculated and incubated anaerobically at 37ºC for 24-48 h (17) while Violet Red Bile Agar (Oxoid CM 107) was inoculated and incubated at 30ºC for 24-48 h for the coliforms (18). For the enterotoxigenic Staphylococcus aureus, Egg Yolk Tellurite emulsion (SR 54) was added into Baird Parker Agar base (Oxoid CM 275) before the inoculation and incubated at 37ºC for 48 h (19), whereas Slanetz and Bartley Medium (Oxoid CM 377) was inoculated and incubated at 37ºC for 48 h for the fecal streptococcus (17). Bacillus Cereus Selective Agar Base (Oxoid CM 617) was inoculated and incubated at 37ºC for 24 h for the Bacillus cereus(15). Perfiringens Agar Base (Oxoid CM 587) was inoculated and incubated at 37ºC for 24 h for the Clostridium perfringens (15). For the mould and yeast, Chloramphenical Antibiotic Supplement (Oxoid SR 78) was added to Rose Bengal Chloramphenical Agar (Oxoid CM 549): inoculated and incubated at 25ºC for 5 days (15). The isolation of Salmonella spp. was performed following the classical methods by using two selective enrichment media of buffered pepton water (37ºC for 24 h) and Rappaport-Vassiliadis broth (41ºC for 48 h) (Oxoid CM 669) (17, 18). Then these cultures were streaked on Hektoen Enteric Agar (Oxoid CM 419), Brillant Green Agar (Oxoid CM 263) and XLD (Oxoid CM 469) (37ºC for 24 h). No further confirmatory tests were performed since no suspected colonies of Salmonella spp. were observed.
Statistical Analysis; ANOVA was performed to analyze the significance between the mean values of the results. The results were considered as significant when p values were less than 0.05.
RESULTS
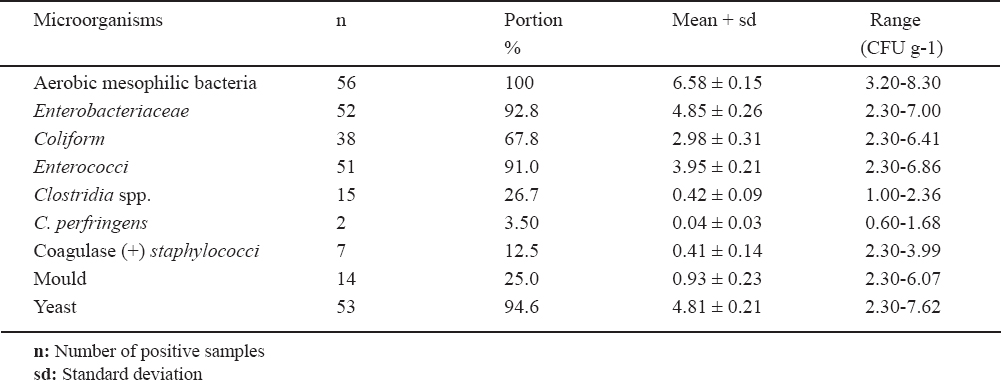
The examination of dried goose carcasses showed that 100% of the samples had aerobic mesophilic bacteria at the average number of log10 6.58 CFU g-1. Yeasts were found in 94.6% of the samples at an average number of log10 4.81 CFU g-1. Enterobacteriaceae and enterococci were enumerated in 92.8% and 91% of the samples at the average counts of log10 4.85 and 3.95 CFU g-1 respectively. Coliforms were detected in 67.8% of the samples at the average number of log10 2.98 CFU g-1 while clostridia were counted in 26.7% of samples at the average number of log10 0.42 CFU g-1. Moulds were counted in 25% of the samples at the average numbers of log10 0.93 and Coagulase positive staphylococci was found in 12.5% of the samples at the average number of log10 0.41 CFU g-1. Clostridium perfringens was isolated from 3.5% of the samples at the average number of log10 0.04 CFU g-1. No Salmonellae and Bacillus cereus were isolated in the samples examined. These results are summarized in Table 1.
Table 1. The mean numbers of microorganisms in dried goose carcasses
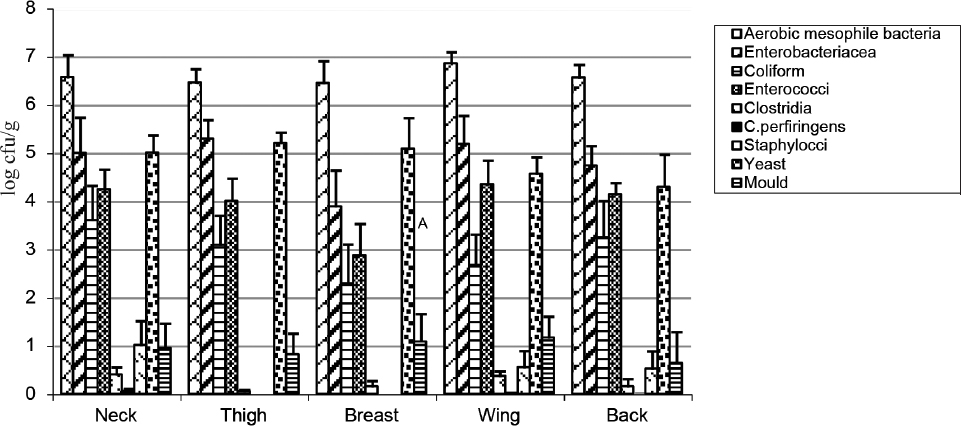
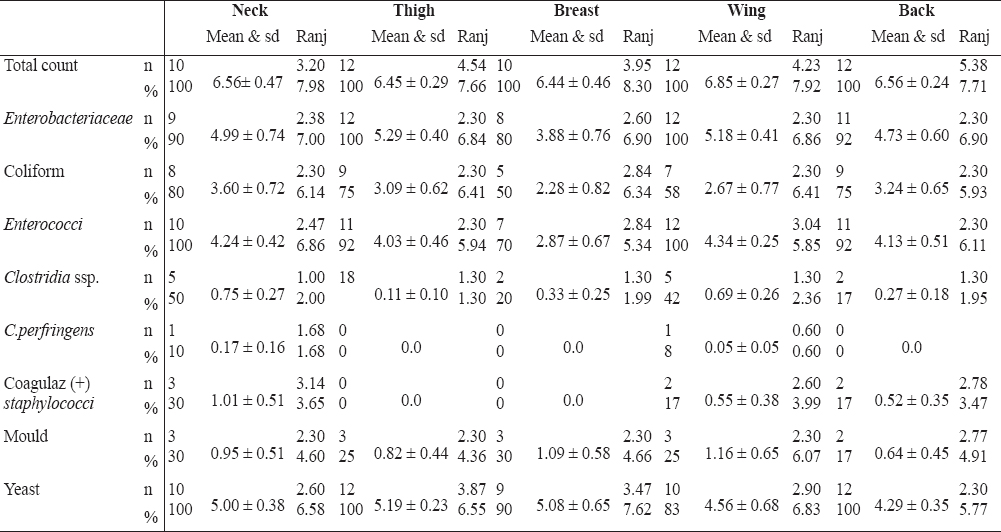
The distributions of the microorganisms in the neck, thigh, breast, wing and back samples of dried goose carcasses are shown in Figure 2 and Table 2.
Figure 2. Distribution of microorganisms in dried goose carcass parts
Table 2. The percentage, range and standard deviations of microorganisms in goose carcass parts
DISCUSSION
The data in the literature indicates that most of the studies about goose focus on the growth performance and carcass characteristics of different goose breeds (20-28). To our knowledge, compared to the other meat types, few studies have been conducted regarding goose meat microbiology and quality in Turkey and in other countries (9, 10, 13, 29-32). This may be probably due to goose meat rather being a seasonal product because of the limited availability of geese at other times of year (30) compared to chicken and red meat, and geese not being raised widely in Turkey and worldwide as a commercial product compared to other poultry. Thus, there is not much relevant data available related to the microbiological quality of goose meat or carcass, especially about the dried goose carcasses, in comparison to the data which exists for chicken or red meat and carcasses.
The microbiological analysis of dried goose carcasses showed that the mean counts of aerobic mesophilic bacteria (log10 6.58) were higher compared to the numbers of 3.14 CFU g-1, log10 5.25, 4.73, 3.67-4.72, 4.51 reported in the chicken carcasses by other authors (33, 34, 35, 36, 37), but similarities were observed with the numbers of 104-106, 6.80 CFU g-1, 106-107, and 6.34-6.7x106 CFU g-1 reported in other studies (38, 39, 40, 41).
The number range of aerobic mesophilic bacteria in our dried goose carcass samples was wide (Table 1), which was also similarly reported in the traditionally processed (dried) raw goose carcass samples they analyzed (9). They reported the mean number of aerobic plate counts as 5x106 CFU g-1 in the range of 103 CFU g-1 to 8.74 CFU g-1. In the study of Xie et al. (31), they monitored spiced geese samples during the production and sale operations and reported that the total aerobic counts significantly (p<0.05) increased and reached up to log10 4.86 CFU g-1 after four hours processing. Considering the undesirable high numbers, the microbial load of goose carcasses may cause to shorten their shelf life and pose a risk to consumers’ health. The high numbers of goose carcasses may also indicate inadequate store of dried carcasses as it was observed in this study.
Enterobacteriaceae were isolated from 92.8% of the goose carcass samples examined at the average number of log10 4.85 CFU g-1. Similar and higher results of log10 4.97 and 2.1×106 CFU g-1 are already reported (42). In comparison to these results, lower numbers of 103-104 CFU g-1 and log10 2.90 were also found in the chicken carcasses by other authors (35, 43). Likewise, in the goose carcass samples, the numbers of Enterobacteriaceae ranged between the numbers of <1.0×102 and ≥109 over the 95% of the samples examined (9). These numbers were higher than the numbers obtained in this study. The presence of Enterobacteriaceae, enterococci and coliform bacteria in the goose carcasses indicates inadequate hygiene or fecal contamination.
Coliform bacteria were detected in the 67.8% of the samples at the average number of log10 2.98 CFU g-1. This result is similar to the results of log10 3.13 CFU g-1 and 1.4x103 (34, 44), but it is lower than the results of 5.1×104 CFU g1 and log10 103-105 investigated in other studies (38, 39). Nair et al. (39) also isolated coliform bacteria from all (100%) the samples they examined. Similar results (33) with the number of log10 2.98 CFU g-1 are compatible with our results. In the study of Guven et al. (9), coliforms were detected in the range of ≥102 and <107 CFU g-1 in the 55% of the goose carcass samples.
In terms of clostridia and Clostridium perfringens, they were isolated from 26.7% and 3.5% of the samples which were quite lower than the values of 102 and 1.01 found in the chicken carcasses in some studies (37, 38). However, in some studies the Clostridium perfringens is not found in some goose carcass samples (9). The level of 26% clostridia in the goose carcass samples showed similarity with the level of 23% clostridia isolated in the broiler samples (43).
Coagulase positive Staphylococcus spp. were isolated from 12.5% of the samples. The average number of log10 0.41 CFU g-1 was found to be similar with the results of log10 <10 (89.79%) and lower than the results reported in the range of ≥102 and <105 (5%) in the goose carcass samples (9). Likewise, it was quite lower than the value of 102 reported in the goose carcasses (45). In other study (31), Staphylococcus aureus was detected in the range of log10 2.65 and 4.89 CFU g-1 in the spiced geese samples indicating that household workshop and the retail outlet were the main place for contamination.
Mould numbers were detected in the range of log10 2.30 and 6.07 CFU g-1 from the 25% of the goose carcasses while the number of yeasts was detected in the range of log10 2.30 and 7.62 CFU g-1 from the 94.6% of the goose carcass samples. Likewise, the numbers of yeast significantly increased in the spiced geese samples (31). The mean numbers of yeasts (log10 4.81 CFU g-1) in our study showed similarity with the mean numbers of yeast (log10 4.05 CFU g-1) reported by the same author in the spiced geese samples (31). When we analyzed the distribution of microorganisms we took into consideration the parts of dried goose carcasses.
Neck samples: Aerobic mesophilic bacteria were found in the neck samples at the number of 1.2x105 whereas aerobic mesophilic bacteria have been found at the average number of log10 6.56 CFU g-1 in the dried goose neck samples. Enterobacteriaceae were isolated from 90% of the goose neck samples at the average number of log10 4.99 CFU g-1 while coliform bacteria were detected in 80% of the neck samples at the average number of log10 3.60 CFU g-1 showing a similarity with the number of coliform bacteria (4.7x103 CFU g-1) counted in the neck samples (46). The presence of Enterobacteriaceae and coliform bacteria in the neck samples indicates inadequate hygiene during slaughtering. Enterococci were isolated in all samples and their numbers changed in the range of log102.47 and 6.86 CFU g-1. Clostridia were detected in 50% of the neck samples at the average number of log10 0.75 CFU g-1. One of these isolates gave positive result for Clostridium perfringens at the number of log10 1.68 CFU g-1 whereas the same authors (46) reported a slightly higher number of log10 1.00 CFU g-1 Clostridia in their chicken neck samples. Coagulase positive staphylococcus were also detected at the average number of log10 1.01 CFU g-1 while other investigators (46) enumerated Staphylococcus aureus in the chicken neck samples three times higher (1.5x103 CFU g-1) than our result. Likewise, coagulase positive staphylococcus was detected at the level of 10.20% in the goose carcasses examined (9). The existence of coagulase positive staphylococcus indicates contaminations during the processes applied to the carcasses. Moulds were found in 10% of the neck samples of dried geese at the average number of log10 0.95 CFU g-1 while yeasts were enumerated at the average number of log10 5.00 CFU g-1 which is higher than the yeast and mould number of 2.8x102 CFU g-1 reported in the neck samples of chicken examined (46).
Thigh samples: Astorga et al. (47) examined chicken thighs and reported the counts of log10 5.56 to 7.28 CFU g-1 aerobic mesophilic bacteria, whereas other authors (38, 42, 46) reported log106.4x105 CFU g-1, 3.37 CFU g-1 and log10 4.6 CFU g-1 numbers of the aerobic mesophilic bacteria, respectively. These results are lower than the average number of log10 6.45 CFU g-1 in the thigh samples of goose carcasses in this study. Likewise, it was fond the numbers of 1.4x106 CFU g-1 aerobic mesophilic bacteria in the chicken thighs (44) but other author (13) reported log10 3.65 CFU g-1 numbers of the aerobic mesophilic bacteria in the goose thigh samples. This is lower than the number detected in this study. Also other researchers reported the aerobic mesophilic bacteria counts of 4.5x107 CFU g-1 and log10 6x107 CFU g-1 in the goose thigh samples (45, 48). These are higher than the number obtained in this study. Enterobacteriaceae were isolated in 100% of thigh samples at the average number of log10 5.29 CFU g-1, while coliforms were detected in 75% of the thigh samples at the average number of log10 3.09 CFU g-1. Likewise, Astorga et al. (47) reported the numbers of log10 3.49 to 5.42 CFU g-1 coliform bacteria in their chicken thigh samples, whereas Kundakci et al. (46) counted the numbers of 3.7x103 CFU g-1coliforms in the chicken thighs. The number of coliforms in the goose thigh samples showed similarity with these results, but higher numbers of coliforms in the chicken thighs have also been reported at the average numbers of 4.1x103 and 1.9x104 CFU g-1 in two studies (38, 49). The lower number of 9.6x102 CFU g-1 coliform bacteria in the chicken thigh samples is also reported (44). This is also lower than the coliform numbers in the goose thighs samples examined in this study. Enterococci were isolated in 92% of the goose thigh samples at the average number of log10 4.03 CFU g-1. This is similar with the result of 1.3x104 (44). Clostridia was detected in only one of the goose thigh samples at the average number of log10 0.11 CFU g-1, whereas other researchers (38, 46) found clostridia at higher numbers of 2.2x102 and log101.00 CFU g-1 respectively in the chicken thigh samples. No coagulase positive stahylococci were detected in the goose thigh samples, same as Ucar et al. (13) who could not isolate any staphyloccus in the goose thigh samples, while other authors (44, 45, 47) isolated staphylococcus at the numbers of 3.6x102 CFU g-1, log10 2.47 to 3.48 CFU g-1 and 6.2x102 CFU g-1 in the chicken thigh samples, respectively. Yeasts were detected in 100% of thigh samples at the average number of log10 5.19 CFU g-1, whereas moulds were isolated only from 25% of the goose thigh samples at the average number of log10 0.82 CFU g-1. In the yeast and moulds it is detected higher number of log10 2.90 in the chicken thigh samples (46). Other authors could not isolate any yeast and moulds from the goose thigh samples (13). This might be due to goose thigh samples being fresh and/or not stored over time.
Breast samples: In the ten goose breast samples examined, aerobic mesophilic bacteria were found at the average number of log10 6.44 CFU g-1. This is higher than the number of log10 3.88 CFU g-1 aerobic mesophilic bacteria found in the goose breast meat samples by Ucar et al. (13) and than the numbers of log10 3.05 CFU g-1, 5.0x104 CFU g-1, log10 5.69 CFU g-1 and in the chicken breast meat samples reported by some researchers (42, 46, 50), whereas others (44, 48) reported aerobic mesophilic bacteria at the numbers of log10 7.00 CFU g-1, and max 3.9x107 CFU g-1 which is higher than these all. Enterobacteriaceae were detected in 80% of the goose breast samples at the average number of log10 3.88 CFU g-1. Other authors enumerated Enterobacteriaceae in the chicken breast-wing samples in the range numbers of 2.0-3.0 CFU g-1 which is lower than the result in this study (50). Half of the goose breast samples contained coliform bacteria at the average number of log102.28 CFU g-1 which is lower than the result of 3.5x103 and 1.4x103 CFU g-1 found in the breast samples (44, 46). Enterococci were isolated from 70% of the breast samples at the average number of log10 2.87 CFU g-1 which is lower than the number of enterococci (2.0x105 CFU g-1) reported by Sagun et al. (44) in the chicken breast samples. Clostridia were isolated from 20% of the goose breast samples at the average number of log10 0.33 CFU g-1. Coagulase positive staphylococci could not be isolated from the goose breast samples. However, some authors (44, 46) reported the presence of staphylococci in the chicken breast samples at the numbers of 1.3x103 and 5.0x102 CFU g-1 respectively. Moulds were isolated from only 30% of the samples at the average number of log10 1.09 CFU g-1 and yeast from 90% of the samples at the average number of log10 5.08 CFU g-1. Gallo et al. (50) found yeast-moulds at the lower numbers of 102-104 CFU g-1 in the chicken breast samples. However, Ucar et al. (13) could not isolate yeast and moulds in their goose meat samples. This could be due to samples being fresh goose meat and/or not stored over time.
Wing samples: In the twelve goose wing samples, aerobic mesophilic bacteria were enumerated at the average number of log10 6.85 CFU g-1. It was reported (13) a lower number of log10 3.82 CFU g-1 aerobic mesophilic bacteria in their goose samples, while some researchers (45, 47, 50), isolated aerobic mesophilic bacteria at the numbers of 6.41 CFU g-1, log10 5.56 to 7.28 and 1.3x108 CFU g-1 in the chicken samples, respectively. These results are lower than our result in this study. Enterococci were detected in 100% of goose wing samples at the average number of log10 5.18 CFU g-1, whereas Gallo et al. (50) reported lower numbers of 102-103 CFU g-1 in the chicken wing samples. Coliform bacteria were counted at the average number of log10 2.67 CFU g-1 in 58% of the goose wing samples. Other investigators (47) counted coliform bacteria in the range numbers of log10 3.49 to 5.42 CFU g-1 which are higher than the result found in this study. Enterococci were detected in all goose wing samples at the average number of log10 4.34 CFU g-1, while clostridia were isolated from 42% of the wing samples at the average number of log10 0.69 CFU g-1. The highest numbers of clostridia were isolated from the wing parts of goose carcasses in this study, but only one of these isolates was identified as Clostridium perfringens at the number of log10 0.60 CFU g-1. Likewise, coagulase positive staphylococci were isolated only from two wing goose samples at the average number of log10 0.55 CFU g-1. However, it is reported that there is no isolate of staphylococci in goose wing samples (13). In other studies (45, 47) Staphylococcus aureus were found at the max. number of 3.48 CFU g-1 and 8.8x102 CFU g-1 respectively, which is quite higher than the number of staphylococci in our study. Moulds were counted at an average number of log10 1.16 CFU g-1 from 25% of the wing samples, while yeasts were enumerated from 83% of the wing samples at the average number of log10 4.56 CFU g-1. On the contrary, Ucar et al. (13) could not detect yeast and moulds in their goose wing samples. On other study (50), the number of yeasts (log10 2.00-4.00 CFU g-1) in the chicken wing samples is higher than the result obtained in this study.
Back samples: The back samples examined revealed aerobic mesophilic bacteria at the number of log10 6.56 CFU g-1. This result shows similarity with the result of log10 6.25 CFU g-1 (50). Enterobacteriaceae were isolated from 92% of the back samples at the mean number of log10 4.73 CFU g-1 which is higher than the numbers of 102-103 CFU g-1 Enterobacteriaceae reported in the back samples by the same researcher (50). Coliform bacteria were isolated from 75% of the back samples at the mean number of log10 3.24 CFU g1. Enterobacteriaceae and enterococci were isolated from 92% of the samples at the mean numbers of log10 0.27 and 4.13 CFU g-1 respectively. Coagulase positive staphylococci and clostridia spp. were isolated from 17% of the back samples at the mean numbers of log10 0.52 and 0.27 CFU g-1 respectively. Moulds were counted at the mean number of log10 0.64 CFU g-1 from 25% of the samples examined. Yeasts were detected in all samples at the mean number of log10 4.295 CFU g-1 were similar to other reported study (50).
Based on all these results and statistical analysis, there are differences between the parts of goose carcasses. Aerobic mesophilic bacteria have been found to be at higher numbers in the neck and wing samples. This makes us think that the carcasses have been dried upside down. In general, aerobic mesophilic bacteria were enumerated at the lowest number of log10 6.44 CFU g-1 in breast samples, while the highest number of log10 6.85 CFU g-1 was detected in wing samples. Enterobacteriaceae were found at the lowest number of log10 3.88 CFU g-1 in the breast samples, while the highest number of log10 5.29 CFU g-1 was detected in the thigh samples. Coliform bacteria were detected the most in the neck samples with the highest number of log10 3.60 CFU g-1, while the lowest number of log10 2.28 CFU g-1 was found least in the neck samples out of the five. Enterococci were counted at the lowest count of log10 2.87 CFU g-1 in the breast samples, whereas the highest number of log10 4.34 CFU g-1 was observed in the wing samples. Enterococci were detected in all wing samples, while their detection was much less in the breast samples. Clostridia spp. were counted less in the thigh samples with the lowest count of log10 0.01 CFU g-1 and the highest count of log100.75 CFU g-1 was found in the neck samples. Clostridia spp. was detected the most in the neck and wing samples compared to the thigh samples. Clostridium perfringens was isolated from one of the neck and wing samples, with the higher number of log10 0.17 CFU g-1 being in the neck sample. Staphylococci could not be detected from the thigh and breast samples, while the highest number of log10 1.01 CFU g-1 was enumerated in the neck samples. Moulds were found less at the lowest count of log10 0.64 CFU g-1 in the back samples, whereas the highest number of log10 1.16 CFU g-1 was found in the wing samples and were isolated the most in the samples of neck, thigh, breast and wing. Yeasts were isolated from all the samples and were counted at the lowest number of log10 4.29 CFU g-1 in the neck samples and the highest number of log10 5.19 CFU g-1 in the thigh samples. Considering the small differences in the counts of yeast in the samples, it is presumed that the dried goose carcasses examined might have been contaminated with yeast during the storage rather than during the processing.
CONCLUSION
In conclusion, the microbiological analysis of dried goose carcasses showed relatively high numbers of bacteria in the goose carcasses in this study. Based on undesirable numbers of 107-108 CFU g-1 in the meat products, the microbial load of goose carcasses may indicate inadequate handling and storage of dried carcasses, and cause to shorten their shelf life. The presence of Enterobacteriaceae, enterococci and coliform bacteria in the goose carcasses indicates inadequate hygiene and/or fecal contamination which require attention in terms of posing a risk to consumer’s health.