INTRODUCTION
Medicinal plants contain diverse phytotherapeutic classes of bioactive compounds such as polyphenols, tocopherols, alkaloids, etc. that hold promise as chemotherapeutic agents (12, 19). Studies have shown that flavonoids exhibit pharmacological activities, some of which include: vasoprotection, anti-carcinogenic, anti-microbial, anti-inflammatory and anti-proliferative effects (12). These properties contribute to the health benefit effects of phytochemicals in herbal plants. The health benefit effects of flavonoids have been attributed to their ability to act as antioxidants, free radical scavengers and quencher of singlet and triplet oxygen, as well as inhibitors of peroxidation reactions (20).
Garcinia kola is known to be very rich in flavonoids (1, 24). Chemical analysis of the seed revealed the presence of Garcinia biflavanones (GB), xanthones, triterpenes and benzophenones (5, 17). The biflavanones are the most dominant in most Garcinia species (24). Kolaviron, an extract from G. kola and a biflavonoid, has also been shown to exhibit antioxidant activity (10). Kolaviron contains biflavonoids GB1, GB2, and kolaflavanone, and has been reported to significantly prevent drug-induced tissue damage in various experimental animal models (9). Bitter kola is eaten raw with folkloric belief that it promotes longevity. Extracts of the plant are used in traditional African medicine for the treatment of laryngitis, cough and liver diseases (15).
In the present study, we investigated the effects of GK on mitogen-induced vascular cell growth. We have evaluated the effects of GK extract on VSMC proliferation, mediated by mitogens such as angiotensin II (Ag II), LPS as well as the mediated regulation of ROS and NO.
MATERIAL AND METHODS
Preparation of the methanol extracts of Garcinia kola seeds
Garcinia kola seeds were purchased from a local market in Ibadan, Nigeria. The seeds were peeled, sliced, pulverized with an electric blender, and dried at 40°C in a Gallenkamp (London, UK) drying oven. The dried seeds were further pulverized with the electric blender and sieved with a fine sieve to give a uniform powder. A known gram/ml of ethanol was subjected to continuous extraction using soxhlet. This extract was then concentrated with a rotary evaporator at 40 - 50°C under reduced pressure to obtain the methanol extract. The dried extract was then stored until it was needed for evaluation.
Experimental design
Cells were cultured to confluence, hypsinized and plated in 96 well plates for cell proliferation assay. Twenty four hours after plating, cells were treated with various concentrations (25-100 µg/ml) of the extract along with the control in the presence or absence of mitogens Ag II or LPS and cultured for 24-96 hours to determine effects of treatment on cell growth. MTT assay was performed at 24, 48, 72 and 96 hours. MTT assay is based on the ability of the cell to reduce MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide to purple formazan in the mitochondria of living cells. The viable VSM cells were seeded at a density of 5 × 104 (100 µL/well) in 96-well plates and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C for 24h to form a cell monolayer. MTT assay was performed over three days. On day one, the VSM cells were trypsinized after these have confluence and on the second day the cells were treated with GK and the final volume of the media was adjusted to 100µl and the incubation continued. On day three 20 µl of 5 mg/ml of MTT was added to each of the 96 wells, but the well used as controls had no cell. This was then incubated for three and half hours at 37°C in culture hood. After this, the media was carefully removed and 150 µl of MTT solvent was added and covered with tin foil and cells agitated on orbital shakers for 15 minutes. Thereafter, the absorbance was read at 590 nm (26). Griess assay was used to measure the Nitric oxide (NO) production according to the method of Olaleye et al. (22). 100 mL of sample were incubated with 100 mL of Griess reagent (Sigma) at room temperature for 20 min. Nitrite level was determined by measuring the absorbance at 550 nm using a spectrophotometer.
Statistics
All values were expressed as mean ± S.D. The test of significance between two groups was estimated by Student’s t test. “One-way ANOVA with Dunnett’s post-test was performed using GraphPad Prism version 4.00.
RESULTS
Cell viability test
The results of cell viability tests are as shown in Figures 1-6.
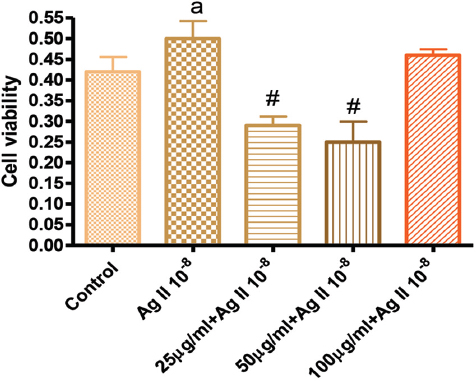
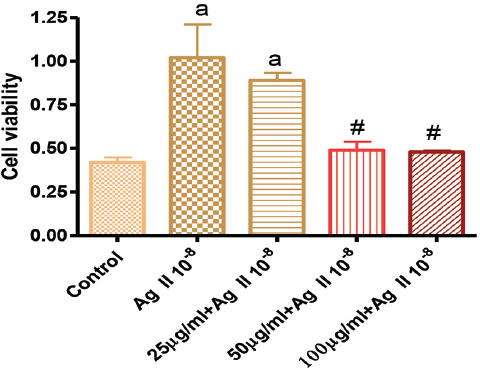
Figure 1. Effects of GK on Angiotensin 10-8 induced VSMC proliferation at 24 hours
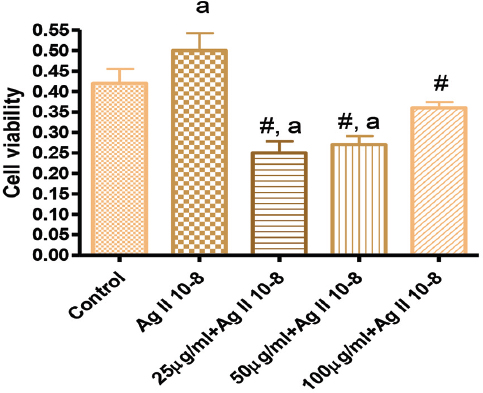
Figure 2. Effects of GK treatment on Angiotensin 10-8 induced cell proliferation at 48 hours
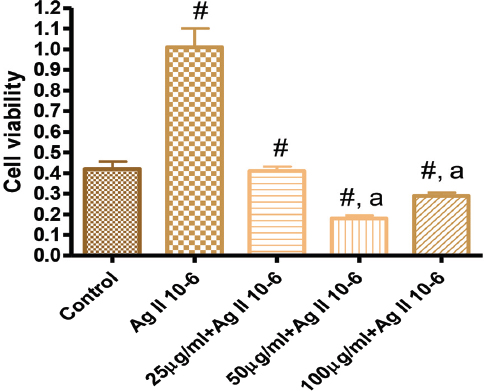
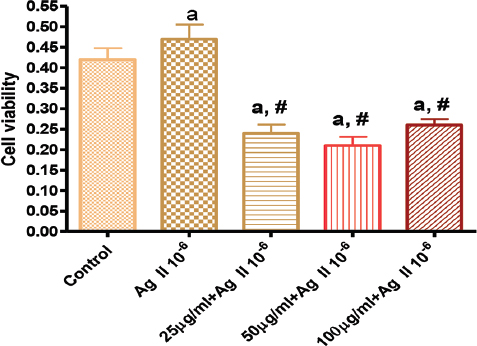
Figure 3. Effects of GK on Angiotensin 10-6 induced cell proliferation at 48 hours
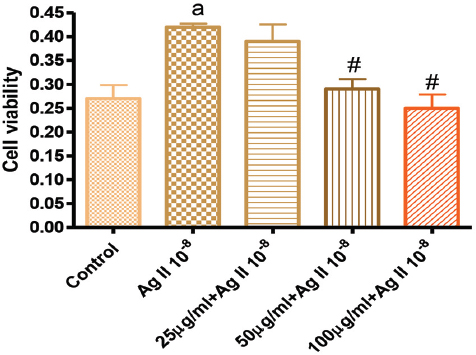
Figure 4. Effects of GK treatment on Angiotensin 10-8 induced cell proliferation at 72 hours
Figure 5. Effects of GK treatment on Angiotensin 10-8 induced cell proliferation at 96 hours
Figure 6. Effects of GK on Angiotensin 10-6 induced cell proliferation at 96 hours
In Figure 1, the effects of GK on Ag II-induced (Ag 10-8) VSMC proliferation showed that 25, 50 µg/ml significantly attenuated cell proliferation, while the 100 µg/ml did not. Figure 2 showed the effect of GK on Ag II (Ag 10-8)-induced cell proliferation at 48 hours. While AG II treatment of VSMC increased cell proliferation compared to the control, this increased proliferation was attenuated by about 50% for 25 µg/ml GK, 40% for 50 and 25% for 100 µg/ml. These attenuations were significantly different from Ag II (Ag 10-8) alone.
Figure 3 showed the effect of GK on Ag II (Ag 10-6)-induced increase cell proliferation at 48 hours. Ag II treatment of VSMC significantly increased cellular growth by 120% compared to the control. This increased proliferation was attenuated by treatment with GK 25-100 µg/ml.
Figure 4 showed that treatment of VSMC with Ag II (Ag 10-8) significantly increased cell growth. However, treatment with GK 25 µg/ml had no effect on Ag II-induced cell proliferation, but GK at 50, 100 µg/ml significantly attenuated Ag II-induced cell proliferation.
Figure 5 showed that at 96 hours of growth, Ag II (Ag 10-8) significantly increased cell growth and this growth was significantly reduced in the presence of GK 50 and 100 µg/ml, but not 25 µg/ml. In figure 6, 96 hours after treatment with Ag II (Ag 10-6), increased cell proliferation was observed, but treatment with GK at all concentrations significantly prevented the Ag II-mediated cell proliferation.
LPS-induced ROS production
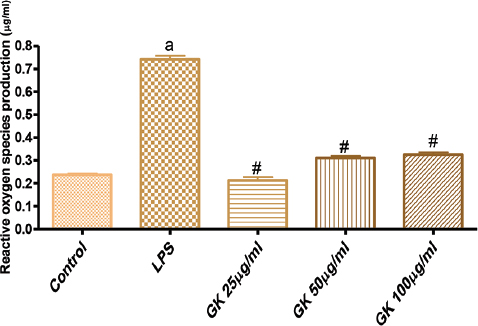
Figure 7 showed that treatment of VSMC with LPS, a pro-oxidant agent significantly increased the production of reactive oxygen species (ROS). Co-treatment of cells with GK (25-100 µg/ml) significantly reduced the LPS-induced increase in ROS production.
Figure 7. Effects of GK on Lipopolysaccharide (LPS)-induced reactive oxygen species (ROS) production
LPS-induced nitric oxide production
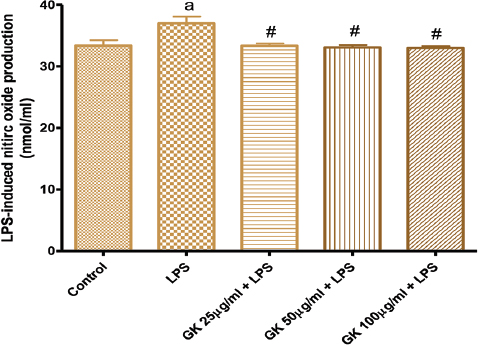
Figure 8 also showed the effects of treatment of cells with LPS on NO production. LPS moderately increased NO production and this production was attenuated and reversed to NO level comparable to the control following co-treatment with GK 25-100 µg/ml.
Figure 8. Effects of GK on Lipopolysaccharide (LPS)-induced nitric oxide production
DISCUSSION
In this study, the methanol extract of GK significantly inhibited VSMC growth. The extract also inhibited LPS and Angiotensin II-induced increased cell proliferations. LPS-induced increased production of ROS, as well as LPS-induced NO productions were also significantly attenuated by GK. The results indicate that GK may possess antiproliferative, antioxidant and possibly anti-inflammatory properties. GK seed is known to be rich in some phytoactive substances such as biflavonoids, xanthones, and benzophenones (17). These active ingredients in GK extract have anti-oxidant, anti-proliferative and anti-inflammatory properties (10). Vascular smooth muscle proliferation has been the hallmark of cardiovascular diseases especially in the development of hypertension, arteriosclerosis. Proliferation leads to intima formation in the vascular system (4, 7, 21). In the present experiment, GK treatment prevented mitogen-induced VSMC proliferation because treatment of VSMC with GK significantly reduced cell proliferation. This effect was time-dependent, as the assay conducted after 96 hours incubation showed a higher inhibition of cell viability profile compared to the other incubation hours. Treatment of VSMC with angiotensin concentrations resulted in increased cell proliferation and this was inhibited by the extract at all the concentrations.
In this study, angiotensin was used as mitogen to promote cell proliferation. Garcinia kola was used to deter some of the effects on angiotensin II-induced proliferation. This result indicates that GK has antiproliferative properties as indicated by its reduction of angiotensin II-induced proliferation, suggesting the possibility of its use in cardiovascular diseases and this has been supported by several scientific reports (2, 8, 9, 11, 15, 16). Angiotensin II has been implicated in a series of disease conditions, especially cardiovascular diseases such as hypertension, stroke, and renal injury. The actions of angiotensin in the aetiology of these diseases are mediated via several pathways which involve generation of free radicals with its effects mediated by stimulation of smooth muscle cell growth, leading to intima formation which are commonly observed in hypertension cases (3, 13). Oxidative stress resulting from ROS has been associated with degenerative conditions such as cardiovascular disease, cancer, weakening of immune system and aging (3). Epidemiological as well as experimental studies have shown that dietary flavonoids may protect against these diseases (14, 18). Most flavonoids exhibit a wide range of biological activities such as antibacterial, antiviral, anticarcinogenic, anti-inflammatory and antioxidant properties (23, 25).
Effect of GK on lipopolyssacharide (LPS)-induced ROS generation was investigated following co-incubations of GK with LPS. The LPS-induced ROS generation was attenuated by the presence of GK. This result suggests that GK possessed an anti-oxidant property. LPS actions on biological systems are also mediated via induction of iNOS-production of NO, along with ROS generation. Also, treatment of cells with LPS along with GK resulted in inhibition of LPS-induced NO production. LPS mediates INOS expression through sequential activation of free radicals, protein kinase C (PKC) and protein tyrosine kinase (PTK) (6). PKC mediates essential cellular signals required for activation, proliferation, differentiation and survival and may be activated through a variety of agonists that may result in free radical generation (27). The GK extract significantly reduced ROS production indicating that the extract is a scavenger or prevents the activation pathway for the generation of ROS. Nitric oxide (NO), which is produced by nitric oxide synthases, plays a major role in a variety of mammalian biological processes including blood pressure homeostasis, immune regulation, and nervous system signal transmission. The concentration of NO determines whether NO acts as a beneficial signal molecule or when overproduced, acts to cause various stress symptoms due to the production of other nitric oxide species, especially peroxynitrite. In this study, LPS-induced nitric oxide production via activation of iNOS was inhibited by the extract indicating that GK can prevent the possible activation of iNOS along with ROS.
CONCLUSION
It could then be concluded from this study that GK may possess anti-inflammatory and anti-oxidant properties due in part to its ability to oppose LPS activities in terms of iNOS inhibition, PKC suppression as well as inhibition of COX-2 expression. The GK seed is eaten raw by humans with the folklore belief that it promotes longevity. The study may have supported this assertion of the seed promoting longevity.