INTRODUCTION
Ciprofloxacin is a fluorinated quinolone with a broad antimicrobial spectrum and high bactericidal activity. It is highly effective against a variety of bacteria – Gram-negative, including Pseudomonasand Enterobacteriaceae, as well as some Gram-positive organisms and many anaerobes and facultative anaerobes. In-vitro tests of the effects of different quinolones against some bacterial pathogens in veterinary practice demonstrated that it is most efficient against Gram-negative microorganisms and mycoplasmae (1, 2). The pyperazine ring to position 7 of the ciprofloxacin molecule is responsible for its activity against Pseudomonas, while the fluorine atom at position 6 confers activity against some Gram-positive bacteria (3, 4).
The bactericide activity of ciprofloxacin, similarly to other fluoroquinolones, is due to the effect on bacterial DNA gyrase (5). The drug suppresses DNA gyrase through interaction with microbial DNA (2).
Ciprofloxacin is well tolerated by animals after parenteral application (6-13). In the organism, it is metabolised in the liver. In humans, there are fiur ciprofloxacin metabolites (sulfociprofloxacin, oxociprofloxacin, desethylenciprofloxacin and formyl-ciprofloxacin), which are excreted with the bile. In animals, it is converted only to two metabolites − desethyl-eneciprofloxacin and oxociprofloxacin, detected in the urine of calves and monkeys (6, 14).
The possible use of ciprofloxacin for treatment of infections, provoked by Mycoplasma and secondary infections (respiratory colibacillosis and pasteurellosis) in birds and pigs requires detailed information about the pharmacokinetic behaviour – distribution, metabolism, elimination and bioavailability of ciprofloxacin in food-producing animal species. Therefore, the purpose of the present experiment was to follow up the pharmacokinetics of this fluoroquinolone in broiler chickens after intravenous and intraingluvial application.
MATERIAL AND METHODS
Animals and housing
The present pharmacokinetic experiment was conducted with 10 sexually mature Ross broiler chickens from both sexes, 60 days of age, weighing 2.75 to 2.840 kg. They were reared in compliance with the standards for the species and fed a compound feed for stock layers. The ambient temperature in the premise was 22-23°C, with continuous ventilation and mixed lighting regimen: 12-hour light period between 8.00 AM and 8.00 PM. The birds were floor reared, and food and water were provided ad libitum. Before the beginning of the pharmacokinetic trial, the birds were neither vaccinated nor treated with other drugs.
Drugs
In this study, the substance ciprofloxacin hydrochloridum was used (Actavis Ltd, Sofia, Bulgaria), dissolved ex tempore in sterile distilled water to obtain 5% solution for intravenous and 1% solution for oral (intraingluvial) application.
Experimental design
The tested fluoroquinolone was applied once, intravenously or intraingluvially at a dose of 10 mg/kg of body weight after preliminary dissolution in sterile distilled water. Intravenous solutions were warmed to 37°C, whereas the solution for oral use was at room temperature.
For intravenous application, solutions were injected in the wing vein (v. brachialis) of the right wing of chickens, and those for oral application were introduced in the crop by means of an elastic silicone probe and a syringe. The intraingluvial application was performed after a 16-hour fasting period (from 4.00 PM the day before to 8.00 AM on the test day). Food deprivation continued for another 6 hours after the crop administration of the drug. Water was withheld for 4 h (1 h before ciprofloxacin application and 3 h after that). A 2-week wash-out period was allowed between the two routes of application of the quinolone.
Care and handling of animals were performed in accordance with the provisions for welfare of Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 adopted by the Government of the Republic of Bulgaria with Ordinance No 20 from 01.11.2012.
Blood sampling
Blood samples of 1 mL were collected from the left wing’s v. brachialis before drug application and at post treatment hours 0.085, 0.17, 0.33, 0.50, 1, 2, 3, 4, 5, 9, 12 and 24. The serum was separated by centrifugation at 1500×g for 10 min. Blood serum samples was stored frozen at –25°C over 3 days until analysis.
Analysis of samples
For assays, ciprofloxacin hydrochloridum substance purchased from Actavis Ltd – Sofia was used, as well as acetonitrile HPLC grade (Merck); triethylamine (TEA; Merck); tetrabutyl ammonium hydrochloride 40% (TBA, Merck); phosphoric acid 85% (Merck) and perchloric acid for analysis 70% (Merck). The water used in the analyses was treated through a Millipore purifying system.
Chromatography system
Serum ciprofloxacin concentrations were assayed by HPLC with UV detection according to the slightly modified method of Imre et al. (15). The difference consisted in substituting blood serum for plasma. The chromatography was run on HPLC system Waters equipped with quaternary pump, HPLC with UV detector, protected and analytical columns Lichrospher (Beckman).
The mobile phase consisted of acetonitrile and water (1:1), supplemented with 3% potassium phosphate buffer, 2% TEA and 2% TBA. Mobile phase pumping rate was 1.2 ml/min. Chromatograms were integrated with a computer program.
The reference standard of ciprofloxacin was used to prepare a stock solution, which was used to fortify serum from untreated chikens (control serum).
The calibration curve for ciprofloxacin consisted of 7 standard solutions that ranged from 0.05 to 10 μg/mL and included a blank (0 μg/mL) sample. The calibration curve was accepted if the linear coefficient of determination (R2) was ≥ 0.99 and if the calibration curve concentrations could be back-calculated to within < 15% of the true concentration of the standard. All serum, calibration, quality-control, and blank serum samples were prepared in an identical manner.
Preparation of samples
Blood proteins were precipitated by consecutive addition of two acids – 85% phosphoric acid and 70% perchloric acid to 100 μL serum, homogenisation by a vortex mixer and centrifugation at 1500×g for 10 min. One hundred μl aliquots of the supernatant were injected in the HPLC system.
Assay validation
Serum ciprofloxacin concentrations were calculated from standard curves from spiked matrix standards. The method of external calibration curves was used for assay validation.
The limit of quantitation (LOQ) for ciprofloxacin was 0.035 μg/mL, and the limit of detection (LOD) − 0.005 μg/mL. The precipitation of serum proteins did not alter the recovery of ciprofloxacin. Intra-day and inter-day coefficients of variation of the method ranged between 5.9–9.19%, and 6.7–12.1%, respectively.
Pharmacokinetic analysis
The TopFit, v. 2.0. software was used for pharmacokinetic investigation of serum concentration time curves after single intravenous or intraingluvial application (16). For description of drug behaviour, two pharmacokinetic models were used –compartmental and non-compartmental analysis (17). The pharmacokinetic modelling was done according to Akaike’s criterion (18). Pharmacokinetic parameters were presented as means (Means) ± standard error (SE). The absolute bioavailability (F) after intramuscular injection of fluoroquinolone solution was calculated as per the equation:
F (%) = [(AUC0→∞p.o. X Di.v.)/(AUC0→∞i.v.. X Dp.o..)] X 100.
Mean absorption times (MAT) were calculated by the formula:
MAT = MRTp.o. – MRTi.v. (16).
RESULTS
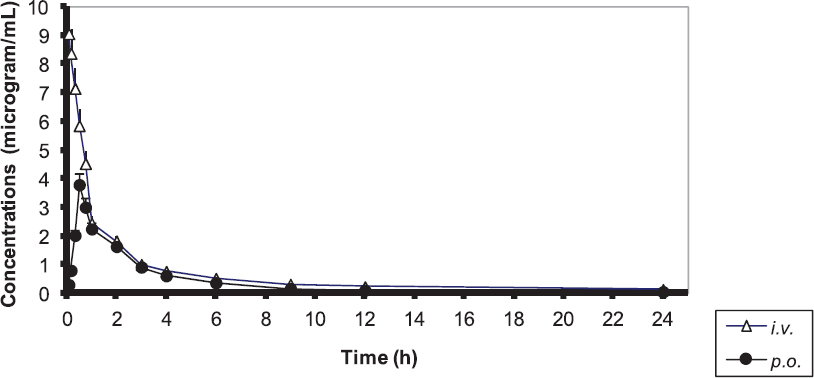
The mean serum ciprofloxacin concentrations assayed after single intravenous or intraingluvial application to broiler chickens are presented on Fig. 1. After the i.v. injection of the aqueous solution of the drug, serum levels remained higher than 0.24 μg/mL up to the 24th h (Fig. 1).
Figure 1. Serum ciprofloxacin concentrations after single intravenous (i.v.) or intraingluvial (p.o.) application to broiler chickens
After the intravenous application, ciprofloxacin concentrations in 8 treated broilers exhibited a biexponential relationship with time, with an initial distribution phase (α) (approximately up to 0.75 h), followed by a slower elimination phase (β) and a long elimination half-life (Table 1). For the other two birds (both of them male), drug concentrations were monoexponential with respect to time.
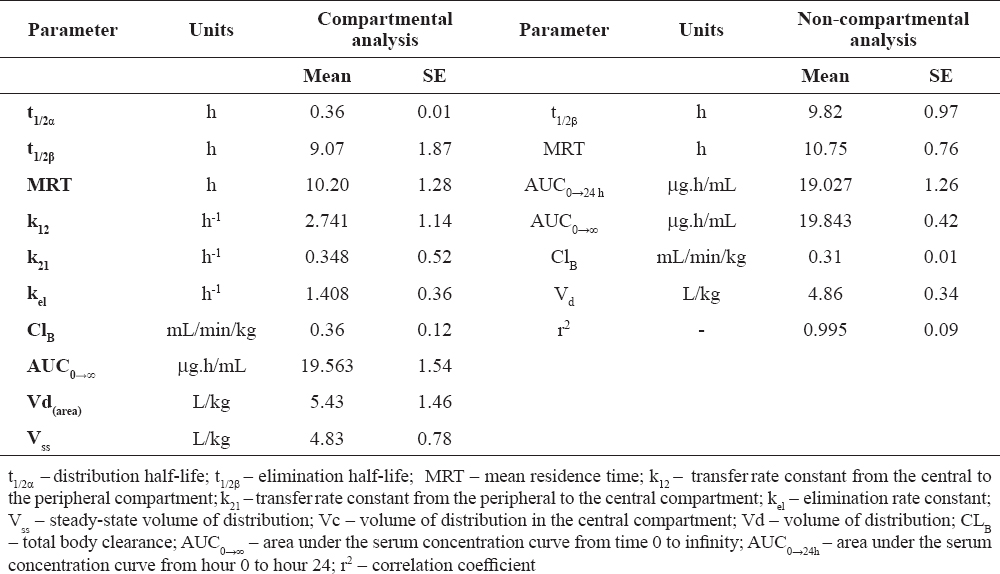
Table 1. Selected pharmacokinetic parameters of ciprofloxacin after single intravenous application to broiler chickens at a dose of 10 mg/kg of body weight (mean ± SE)
Serum concentrations after the intraingluvial application of the tested fluoroquinolone were detectable as early as the first blood sampling – by hour 0.08 and were over the limit of quantification (LOQ) until the 24th hour (Fig. 1). The parenteral application of 5% solution of the drug resulted in rapid attainment of maximum serum concentrations (Cmax) – after about 20-25 min. Intraingluvially treated birds exhibited biexponential reduction of fluoroquinolone levels in all birds, rapid distribution (α phase) and prolonged elimination (β phase).
The rate of absorption of ciprofloxacin in intraingluvially treated birds is presented in Table 2 and were characterised by pharmacokinetic parameters t1/2abs. and MAT.
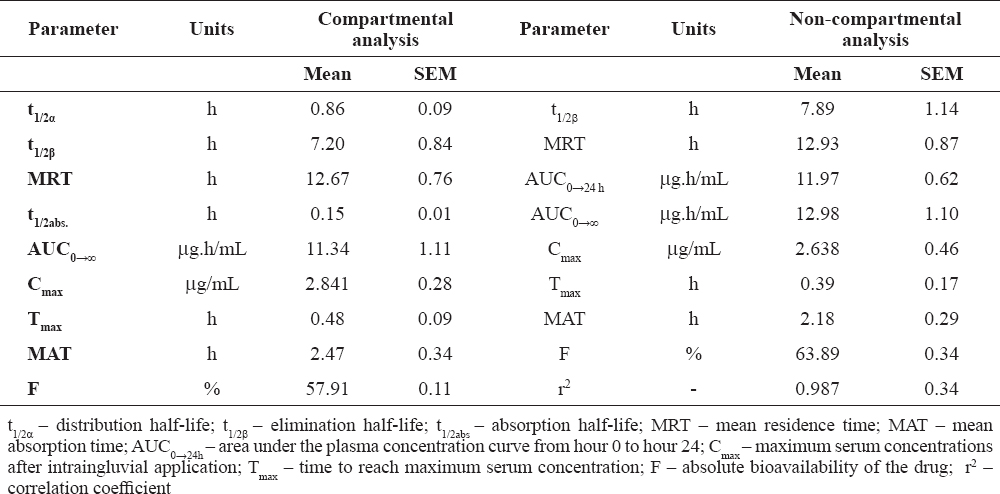
Table 2. Selected pharmacokinetic parameters of ciprofloxacin after single intraingluvial application to broiler chickens at a dose of 10 mg/kg body weight (mean ± SE)
DISCUSSION
In this study, serum ciprofloxacin concentration and the pharmacokinetic behaviour of the tested fluoroquinolone were established in adult clinically healthy broiler chickens. It should be emphasized that after intravenous application to broiler-type birds, the drug exhibited an initial rapid distribution phase (up to 0.36 h, followed by a continuous elimination phase. This finding is in agreement with results reported by other researchers on the administration of this antibiotic in different broiler chicken strains at various dose rates (9, 11, 12, 19). Similarly to others’ reports (9, 12) in 8 out of the ten i.v. injected chickens, serum curves were well fitted to a two-compartmental open pharmacokinetic model. In injected broiler chickens, ciprofloxacin was characterised with a long elimination period, as evidenced by elimination half-life of the drug (t1/2β) and mean residence time (MRT) values calculated by both used pharmacokinetic models (compartmental and non-compartmental analysis) which were higher than data obtained in broiler chickens by García Ovando et al. (t1/2β = 3.11 ± 0.25 h), Raina et al. (t1/2β = 5.79 ± 1.14 h), Anadón et al. (t1/2β = 8.84 ± 2.13 h), but similar to those of Atta and Scharif (t1/2β = 9.01 ± 0.79 h) (9, 11, 12, 19). The longer elimination half-life (t1/2β) in i.v. treated broiler chickens in our experiment was probably due to the different used ciprofloxacin dosage. It is obvious that if concentration is higher, then the elimination process is longer.
The area under the concentration time curve (AUC0→∞) after i.v. administration of ciprofloxacin to broiler chickens (Table 1) was higher than those reported by Raina et al. (AUC0→∞ = 4.54 ± 0.59 μg.h/mL), García Ovando et al. (AUC0→∞ = 5.67 ± 0.52 μg.h/ml), Anadón et al. (AUC0→∞ = 17.71 ± 4.75 μg.h/mL) and lower than that observed by Atta and Scharif in chickens (AUC0→∞ = 78.04 ± 9.45 μg.h/mL) (9, 11, 12, 19).
It was demonstrated that after intraingluvial application, ciprofloxacin was rapidly absorbed from the digestive tract of chickens, as indicated by the values of t1/2abs., Tmax and Cmax (Table 2). Our values are comparable to those published by other research teams (9, 12, 20).
In this pharmacokinetic study, the elimination half-life (t1/2β) and the mean residence time (MRT) (Table 2) following intraingluvial application of the fluoroquinolone, determined by both pharmacokinetic models − compartmental and non-compartmental analysis − were longer than those reported by Raina et al. (t1/2β = 4.37 ± 0.36 h and MRT = 6.17 ± 0.49 h) (12), and similar or close to data of Anadón et al. (t1/2β = 11.89 ± 1.95 h; MRT = 13.32 ± 2.65 h) (20). Hypothetically, the differences vs Raina et al. could be attributed to the different applied doses and experimental design, as well as to the different broiler chicken strains used (20).
The absolute bioavailability (F) of the tested fluoroquinolone after oral administration into the crop, was lower than that reported by Anadón et al. (F = 70.09 ± 9.8%) in broiler chickens treated via the same route at 8 mg/kg (12).
When evaluating data after the single crop application of ciprofloxacin to 2-month-old broiler chickens, the presence of individual antimicrobial drug concentrations > 0.1 μg/mL between post treatment hours 0.08 and 12 deserves to be pointed out, as it contributes to a 12-hour antimicrobial activity higher than MICs of a number of clinically important microbial pathogens for this poultry species.
ETHICAL APPROVAL
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the study was conducted. The experimental procedure was approved by the Ethical Committee at the National Diagnostic and Research Veterinary Medical Institute (Protocol No 61-A/19.03.2012).
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.