INTRODUCTION
“Dalmatian turkey” has been traditionally grown at numerous households in Dalmatia hinterland, Croatia, as an indigenous type of poultry important for local community. Extensive fattening of this turkey is of seasonal type as fattened turkeys are generally sold during late autumn and winter, mostly as a Christmas season speciality (1, 2). As stated by Ekert Kabalin et al. (3), the specific rearing type is one of the reasons for the characteristic flavour and high value of the “Dalmatian turkey” meat. These turkeys are kept free range, where they are grazing, and proteins, minerals and vitamins are supplemented in their feed according to the needs of the species and production category. This type of turkey rearing reduces feeding costs and consequently the costs of overall turkey meat production, while simultaneously favouring their well-being. The houses where turkeys are kept during the warm phase of breeding (approximately the first two months of life) and at night are simple, frequently improvised shelters or some old premises redesigned to turkey shelters.
Good air quality in poultry houses is essential for animal health and productivity, as well as for farmer health and nearby environment (4, 5). The air in these settings is frequently contaminated with various pollutants such as noxious gases and microorganisms. Ammonia is a noxious gas generated by bacterial breakdown of organic nitrogen compounds in manure (6). Carbon dioxide is produced by fuel combustion and bird breathing as a metabolic end-product (7) and is considered as sanitary indicator of indoor air quality. It is also released from the manure (8). Considering microorganisms, saprophytic bacteria prevail in poultry houses; however, the presence of pathogenic bacteria, fungi, spores and other microorganisms has also been reported. Nearly 80% of microorganisms present in the air of poultry houses originate from the animals accommodated there (9-11). Air concentrations of microorganisms and noxious gases depend, among others, on bird age, type of housing system and stocking density, manure management, efficiency of ventilation, and microclimate that is primarily determined by air temperature, relative humidity and airflow rate. The concentrations of these pollutants have been increasingly investigated in intensive poultry production (12-15).
In the present case study, the relation between microclimate (air temperature, relative humidity and airflow rate) and air concentrations of noxious gases (ammonia and carbon dioxide) and microorganisms (bacteria and fungi) in the house for extensively reared turkeys in Dalmatia was assessed.
MATERIAL AND METHODS
The study was carried out in a turkey house at a family household in Dalmatia hinterland, Croatia, during the final period of turkey extensive fattening, from September (the end of summer and the beginning of autumn) to December 2014 (the end of autumn and the beginning of winter). Dalmatia belongs to the Mediterranean climate region characterized by hot and dry summer and mild and wet winter.
All study turkeys had been hatched in spring. The house is a stone building redesigned for turkey house accommodating 50.3±3.1 turkeys. The house dimensions are 2.9 m L x 4.2 m W x 2.3 m H, with concrete floor, wooden door (0.8 m W x 2.0 m H) and perches for turkeys. Lighting is natural through window sized 1.5 m W x 1.0 m H. The house was regularly cleaned once a week. During the day, turkeys were grazing free range and supplemented with corn twice a day (in the morning and in the evening) from plastic dishes outside the house. Turkeys had access to tap water ad libitum from improvised dishes indoor and outside the house.
Microclimate parameters, noxious gas concentrations and microbiological contamination of indoor air were measured three times per month, every tenth day, in the morning, prior to releasing turkeys out for grazing. Air temperature (°C), relative humidity (%), airflow rate (m/s), and ammonia (ppm) and carbon dioxide (ppm) concentrations were measured by portable devices (Testo and Dräger, Germany). Air was sampled by use of the SAS 100™ device (PBI International, Italy). Air samples were cultured into Petri dishes with nutrient medium for determination of total count of mesophilic bacteria (Nutrient agar, Biolife, Italy) and fungi (Sabouraud maltose agar, Biolife, Italy), which were then incubated at 37 °C for 24 h for mesophilic bacteria and at 22 °C for 5 days for fungi, as previously described (16, 17). The grown colonies were expressed as number of colony-forming units per m3 air (CFU/m3), with results corrected as per table and equation supplied with the device. On each occasion, all study parameters were measured at the same five sites in the house (in the centre and corners), in turkey biozone, in order to obtain average representative values. Throughout the study period, outdoor air temperature and relative humidity were measured at the same two sites, in front of and behind the house.
The data collected were analysed using the Statistica v. 12.5 (StatSoft Inc., 2014) software. Basic data processing was done by use of descriptive statistics methods. Because of the limited number of measurements per month, differences in study parameters according to months were tested by non-parametric test, Friedman analysis of variance, while Spearman rank order correlation was employed to determine correlations among the parameters.
RESULTS
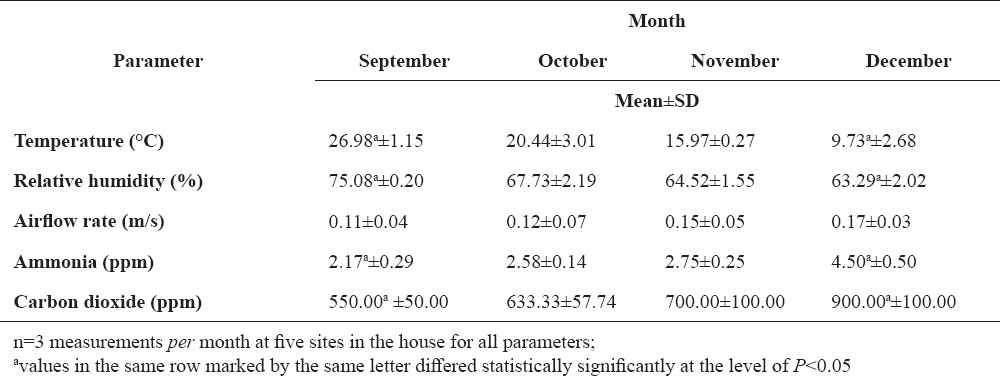
The highest values of air temperature (26.98 °C) and relative humidity (75.08%) in turkey house were recorded in September, differing significantly (P<0.05) from the lowest values recorded in December (9.73 °C and 63.29%, respectively). Airflow rate was highest in December (0.17 m/s) and lowest in September (0.11 m/s), with no significant differences throughout the study period. Indoor air concentrations of ammonia and carbon dioxide were highest in December (4.50 ppm and 900 ppm, respectively) and lowest in September (2.17 ppm and 550 ppm, respectively), with a significant difference (P<0.05 both) between the months (Table 1).
Table 1. Microclimate parameters and noxious gas air concentrations in turkey house during final period of extensive fattening
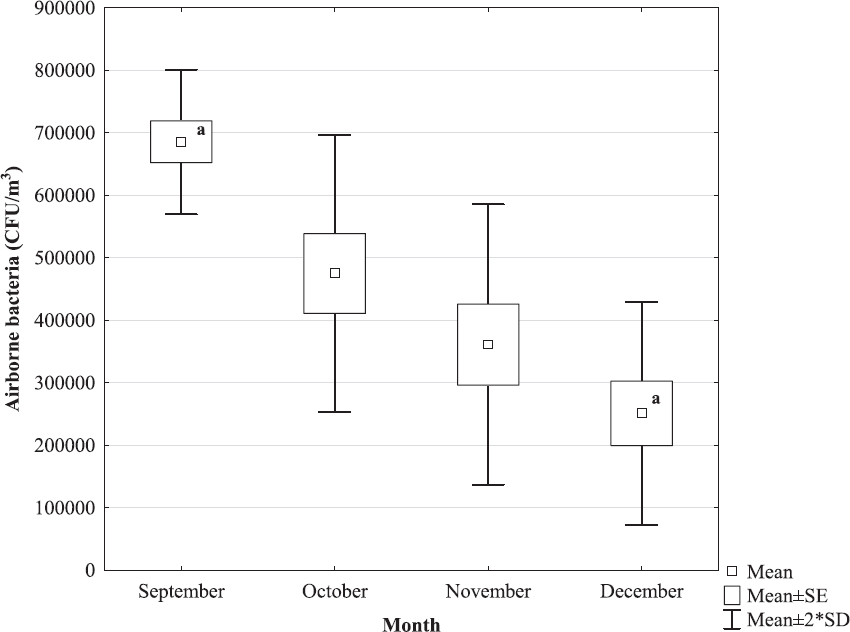
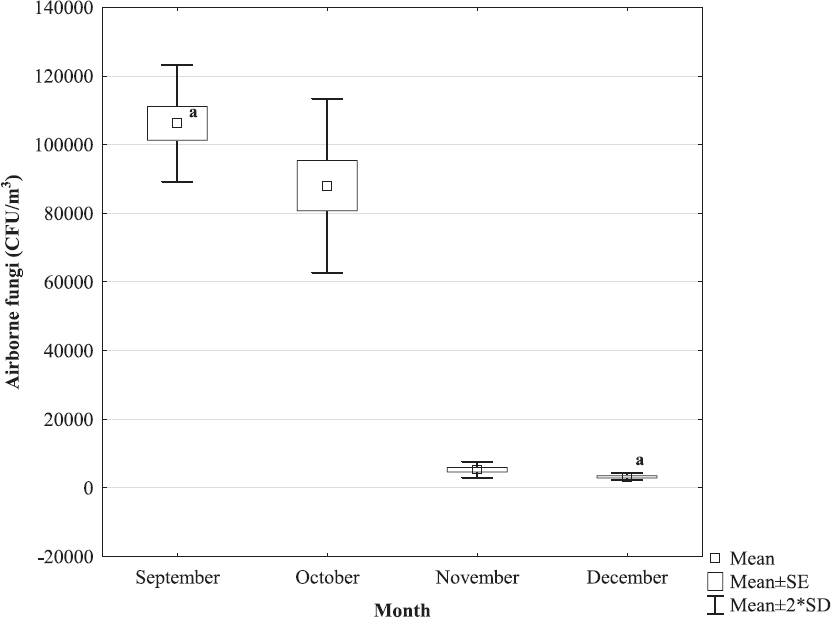
Total bacterial count in indoor air ranged from 2.51x105 CFU/m3 (December) to 6.85x105 CFU/m3 (September) and total fungal count ranged from 3.27x103 CFU/m3 (December) to 1.06x105 CFU/m3 (September), with significantly lower concentrations (P<0.05 both) recorded in December as compared with September (Fig. 1 and 2).
Figure 1. Total bacterial count in the air of turkey house during final period of extensive fattening n=3 measurements per month at five sites in the house;
avalues marked by the same letter differed statistically significantly at the level of P<0.05
Figure 2. Total fungal count in the air of turkey house during final period of extensive fattening n=3 measurements per month at five sites in the house;
avalues marked by the same letter differed statistically significantly at the level of P<0.05
The highest value of outdoor air temperature (27.37 °C) was recorded in September, differing significantly (P<0.05) from the lowest value recorded in December (3.86 °C), while the highest value of relative humidity outside the house was found in December (72.07%) and lowest in October (59.39%); yet, there were no significant differences between the months.
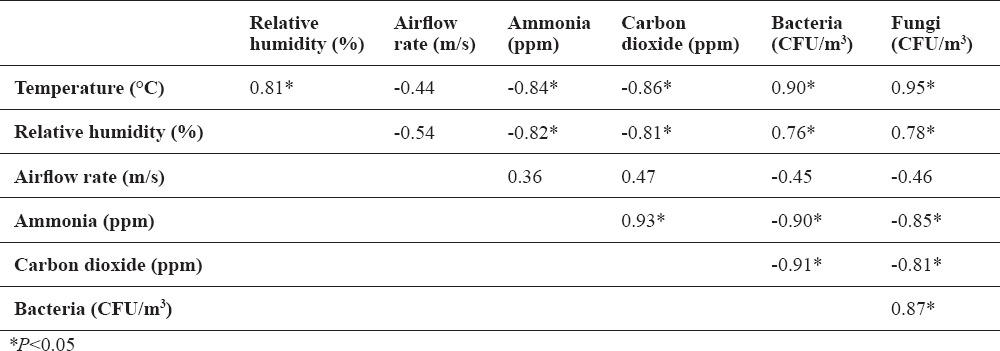
Air temperature and relative humidity inside the house showed negative correlation with ammonia and carbon dioxide concentrations and positive correlation with bacterial and fungal counts in indoor air (P<0.05 all). There were no significant correlations between airflow rate and other parameters observed. Positive correlation was found between ammonia and carbon dioxide concentrations, as well as between bacterial and fungal count in the air (P<0.05 all). Bacterial and fungal count showed negative correlation with ammonia and carbon dioxide concentrations in the air (P<0.05 all) (Table 2). Outdoor air temperature showed positive correlation with indoor air temperature (P<0.05), thus both showing comparable correlations with other parameters observed. Outdoor relative humidity showed no significant correlation with other parameters observed.
Table 2. Spearman rank order correlation among study parameters
DISCUSSION
Intensive turkey fattening implies rearing in confined houses with full feeding and microclimate control. In this type of rearing, optimal indoor air temperature after the warm phase of turkey production is 18-20 °C, relative humidity 60%-75%, and airflow rate 0.1-0.25 m/s. Fattening generally takes 13-16 weeks, when turkeys gain body mass of 6-9 kg. In case of sex specific fattening, female turkeys gain body mass of 5-8 kg in a period of 14-16 weeks, whereas male turkeys gain body mass of 14-16 kg in the period of 20-22 weeks. In case of extensive rearing, fattening generally takes longer period of 24-32 weeks, when turkeys weigh 5-8 kg. Extensive fattening is characterized by keeping turkeys in controlled conditions during the warm phase of rearing, followed by free range keeping till day 150 on average, with supplementary feeding with concentrated feed, and turkey fattening in the house for additional 60 days (18, 19).
The present study included turkeys on extensive fattening, i.e. after the warm phase of rearing they were kept free range throughout the period of fattening whenever possible by weather conditions, and kept indoor at night. The study was performed in the final period of fattening (September-December), with the values of microclimate parameters being within the range recommended for intensive turkey fattening throughout the study, except for air temperature (Table 1).
Tolerance to ammonia is considerably lower in poultry as compared with other animal species. Ammonia concentration of 20 ppm will cause eye and airway irritation in poultry, resulting in impaired feeding, lower weight gain and larger susceptibility to viral infections, thus being considered a critical level of ammonia (20, 21). Carbon dioxide is present in the atmosphere at a concentration of 300-400 ppm, with 3000 ppm as the maximal allowed level in the air of poultry house (11, 22). Higher air concentrations of carbon dioxide will cause circulatory derangements and appetite reduction. Increased air concentration of carbon dioxide is as a rule accompanied by higher ammonia concentration (19), as also confirmed in the present study (Table 2), where the measured concentrations of both gases were several times lower than their critical values for poultry throughout the study period (Table 1).
Generating high ammonia and carbon dioxide concentrations in the air of poultry houses has been associated with higher indoor air temperature, due to higher microbial activity in the manure during warm weather (23, 24). However, in intensive production, their concentrations generally are highest during winter due to reduced ventilation, i.e. lower airflow rate, in order to minimize heat loss (25-27). As reported by Zhao et al. (28), higher ventilation rate associated with warmer indoor air leads to higher rate of manure drying out and thus to lower ammonia production. In cold weather, less intensive ventilation and humid air result in higher manure humidity, which favours microbiological decomposition of uric acid to ammonia.
In our study, the levels of ammonia and carbon dioxide in indoor air were also higher in cold season (Table 1), but there was no significant correlation between ammonia/carbon dioxide concentrations and airflow rate, i.e. airflow was stable during the observed period. In addition, noxious gas concentrations showed negative correlation with microorganism count in the air, air temperature and relative humidity (Table 2). Therefore, the higher concentrations of these gases in the indoor air during cold weather, i.e. at the end of fattening period, could be attributed to the growth and greater body mass of turkeys, as supported by Miles et al. (29). An additional explanation of the increased ammonia and carbon dioxide concentrations in indoor air during cold season may be found in the fact that turkeys probably stayed longer in the house due to shorter daylight and considerably lower outdoor air temperature.
Comparative studies of air quality in the houses of various farm animals have revealed the highest levels of microorganisms just in the air of poultry houses (30-32), with bacterial count in the air of house accommodating turkeys reared in intensive systems reaching up to 108 CFU/m3 and fungal count 104 CFU/m3 (33). The bacterial and fungal air concentrations measured in the present study were within these limits, with significantly lower values recorded in December as compared with September, i.e. during lower air temperatures (Fig. 1 and 2).
avalues marked by the same letter differed statistically significantly at the level of P<0.05The microorganism counts, just like noxious gas concentrations, in the air of houses of intensively reared poultry generally are higher in cold months as compared with summer months, which is attributed to the lower airflow rate in the houses during cold season, as discussed by Matković et al. (17). In the present study, there was no significant correlation between bacterial/fungal count in the air and airflow rate. However, significant positive correlation was found between microorganism count and air temperature/relative humidity (Table 2). Air concentrations of the bacteria and fungi were lower during cold period, which can be explained by the fact that the microorganisms requiring temperature >20 °C for their growth and development were determined in this study. Investigating microbiological air contamination in poultry houses in summer and winter, Wójcik et al. (16) found higher bacterial and fungal counts during cold season; however, the lowest microorganism count in winter was recorded in a house with lowest air humidity and highest air velocity, which is consistent with the present study. Popescu et al. (34) also found positive correlation between bacterial and fungal count in the air of poultry house and relative humidity; as explained by the authors, the bacteria require humidity to stay viable and be air-borne, while the 50%-70% level of humidity is optimal for their destruction. Yet, there seems to be no clear consensus on the impact of humidity on the survival of bacteria and fungi in the air (35).
CONCLUSION
The present case study investigated microclimate conditions, air temperature, relative humidity and airflow rate in the house of turkeys on extensive fattening, and their relation with the concentrations of ammonia, carbon dioxide, bacteria and fungi in indoor air. Study results revealed higher concentrations of noxious gases and lower microorganism count in the indoor air during cold season and at lower air humidity, and vice versa.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.