INTRODUCTION
Several types of stem cells have been proposed for therapies, with the basis of their potential benefit ranging from providing nutritive support for injured host tissue, as a reservoir for growth factors and as replacement neurons for host cells lost to injury or disease. An underlying prerequisite for all of these goals is survival and appropriate localization of the transplanted stem cells (1). The successful use of mesenchymal stem cells in the treatment requires a method to detect transplanted cells in-vivo in order to understand the mechanisms of transplanted cell’s migration, homing as well as the engraftment efficiency and functional capability (2, 3, 4).
Magnetic resonance imaging (MRI) is one of the ideal tracking methods, because it is noninvasive, high resolution and allows tracking in four dimensions including time, though it requires a suitable contrast agent to be loaded to the tracked cells. One of the most commonly used in stem cell tracking is a group of agents known as magnetic nanoparticles (5, 6, 7). Molday ION Rhodamine-B™ (MIRB) is a new superparamagnetic iron oxide (SPIO) contrast agent specifically formulated for cell labelling, which is readily internalized by non-phagocytic cells. It can be visualized by both MRI and fluorescence microscopy and it can assess the potential for imaging and monitoring of MSCs transplantation (8).
MATERIAL AND METHODS
In the present study, oADMSCs were isolated from perirenal fat of sheep according to the method published previously, using type I collagenase (SIGMA ®Cat. No. CO130). The cells were cultured using DMEM with 10% FBS and 1% antibiotic-antimycotic solution. In the primary culture (P-0), 70 % confluency was observed 17 days post incubation. Whereas, in subsequent passages the cells grew at a faster rate and 70 % confluency was noticed in 3 days. Then the oAMSCs were subcultured using 0.25% typsin (9).
Plates of oADMSCs, from passage 4 to passage 6 with 70 % confluency were used for labelling.
Preparation of labelling solution
MIRB (BioPAL Inc.) was added to the medium (DMEM + 10% FBS + 1% antibiotics-antimycotic) at the concentration of 25 µg Fe/ml as per (8, 10). Labelling solution was filtered with syringe filter before use.
Labelling of oADMSCs
The oADMSCs were labelled by culturing the cells in the labelling solution containing 25 µg Fe/ml (8, 10). The culture plates were incubated at 37°C with 5% CO2. The spent medium was replaced by a fresh medium containing MIRB at every 24 hrs (1). The labelled oADMSCs were examined under fluorescent microscope (Olympus) to observe the integration of MIRB (8).
The labelled oADMSCs were assessed for intracellular MIRB distribution, viability and in-vitro MRI tracking.
Intracellular MIRB analysis
The unlabelled and MIRB-labelled oADMSCs (72hrs of MIRB incubation) of P-4, P-5 and P-6 were fixed with biopal fixative and further subjected to Prussian blue staining to demonstrate the iron uptake of the cells (11).
Cell viability
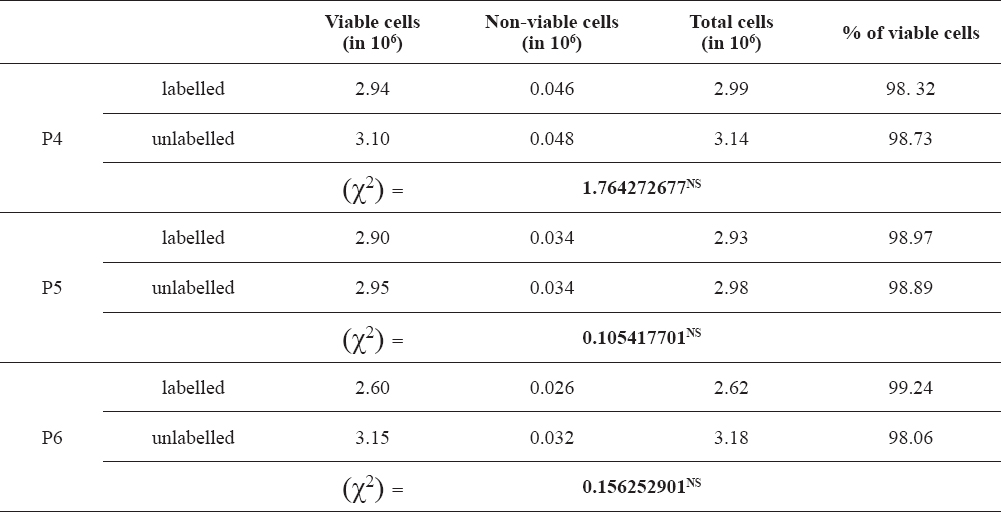
Trypan blue exclusion test was used for assessment of cell viability by using the formula: unstained cells / total number of cells x 100 as per (12). The viability of labelled and unlabelled cells was analyzed statistically by a chi-square test as per standard protocol (13).
In-vitro MRI detection of iron
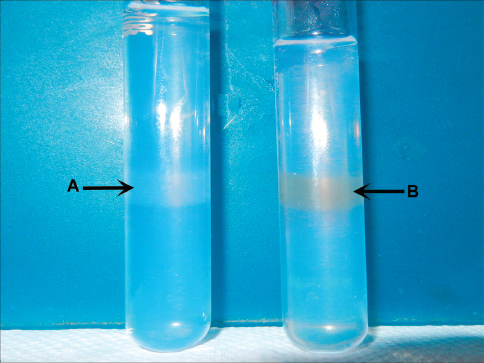
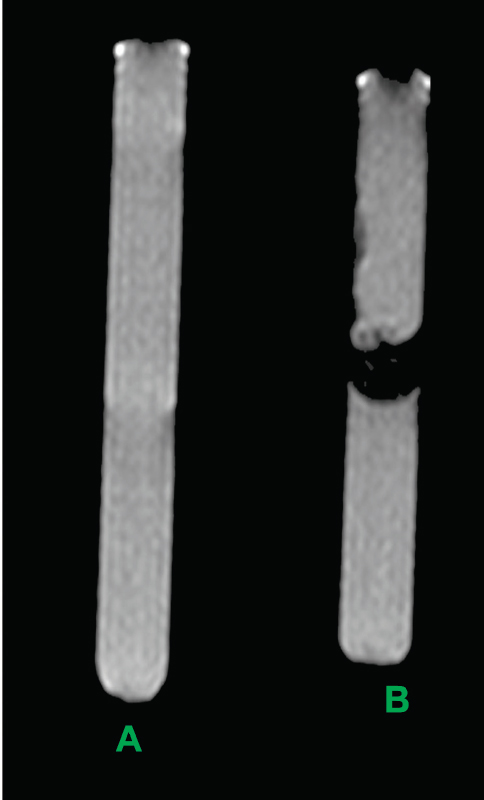
For in-vitro MRI evaluation of MIRB-labelled oADMSCs, phantom models containing MSCs in agar were used. Phantoms were constructed as groups of 10 ml sample tubes (borosilicate glass culture tubes) containing MIRB-labelled oADMSCs suspended in agar. Differences between the MIRB-labelled oADMSCs and unlabelled oADMSCs were studied in MR relaxation time. MIRB-labelled oADMSCs (72 hrs MIRB incubation) and unlabelled oADMSCs were centrifuged into a pellet and resuspended with melted 0.5 % agarose and sandwiched between two layers of 0.5 % plain agar (Fig. 1). Each tube contained 1 × 106 cells. The tubes were imaged with an eight-channel phased-array head coil on a clinical 1.5 T Siemens Symphony MRI unit (8, 14).
Figure 1. Unlabelled (A) and MIRB-labelled (B) oADMSCs sandwiched between two layers of 0.5 % plain agar
RESULTS
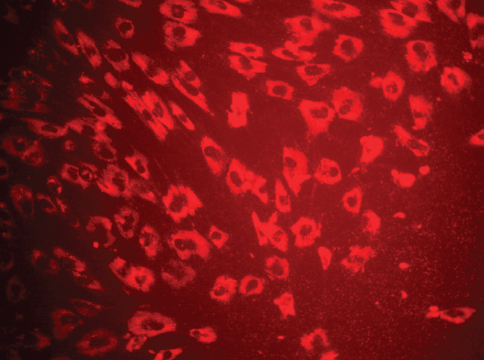
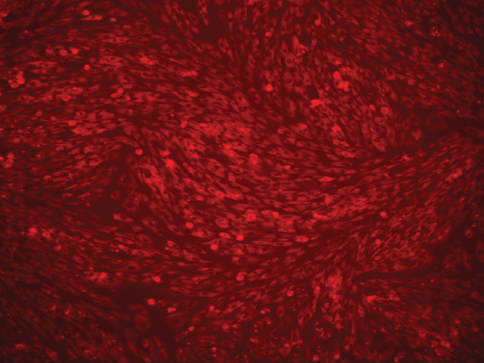
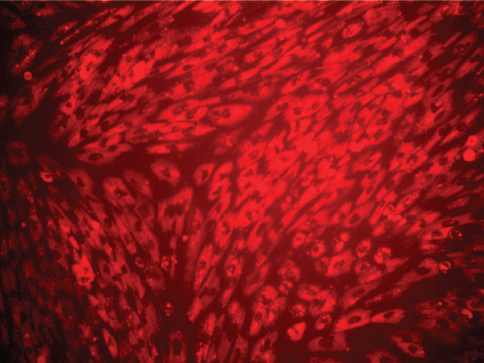
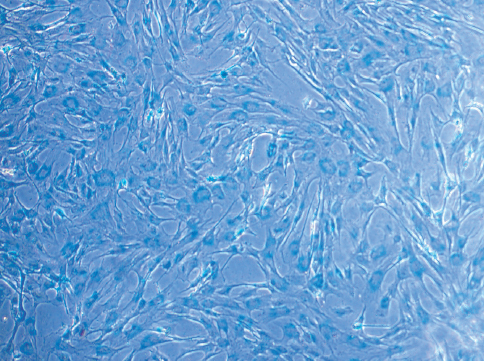
In the present study, no fluorescence of MIRB could be detected within the cytoplasm of oADMSCs of P-4, P-5 and P-6 after 24 hrs and 48 hrs of post incubation. However, 72 hrs post incubation, internalized MIRB was observed as red colored fluorescent clusters in oADMSCs of P-4 (Fig. 2), P-5 (Fig. 3) and P-6 (Fig. 4). This indicated the intracytoplasmic integration of iron nanoparticles after 72hrs of incubation.
Figure 2. Micrography of MIRB labelled ADMSCs (P-4 level) 72 hrs of post incubation. MIRB; bar = 200 x
Figure 3. Micrography of MIRB labelled ADMSCs (P-5 level) 72 hrs of post incubation. MIRB; bar = 200 x
Figure 4. Micrograph of MIRB-labelled ADMSCs (P-6 level) 72 hrs of post incubation. MIRB; bar = 200 x
Figure 5. Micrograph of MIRB-labelled oADMSCs showing positive intracytoplasmic blue inclusion. Prussian blue; bar = 200 x
Figure 6. MRI image of unlabelled (A) and MIRBlabelled (B) ADMSCs sandwiched between two layers of 0.5 % plain agar
Intracellular MIRB analysis
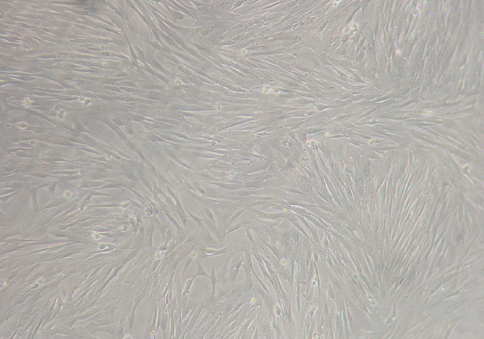
The MIRB-labelled oADMSCs of passages 4, 5 and 6 showed positive intracytoplasmic inclusion blue reaction to Prussian blue (Fig. 5), whereas unlabelled cells did not show positive reaction (Fig. 7).
Figure 7. Micrography of unlabelled ADMSCs (Control) 72 hrs of post incubation; bar=200 x
Viability assay
In the present study, 98.4 % of oADMSCs were viable after 72 hr incubation with MIRB in P-4. It was observed that 98.8 % cells in P-5 and 98.9 % cells in P-6 were viable in 72 hrs of incubation (Table 1).
Table 1. Viability in MIRB labelled oADMSCs
No significant difference was observed between the viability of MIRB-labelled oADMSCs and unlabelled oADMSCs (Table 1). The viability of the MIRB-labelled oADMSCs ranged between 98-99 %.
MRI
In the present study, MR signal intensity was lower in MIRB-labelled ADMSCs (P-4, P-5, P-6) when compared to control (unlabelled) group of cells (Fig. 6). No significant difference was observed based on MR signal intensity between the oADMSCs at P-4, P-5, P-6.
Emission of MR signals in labelled cells indicated the successful bio-imaging at cellular level in visualizing oADMSCs in-vitro.
DISCUSSION
Intracellular MIRB analysis
In the present study, intracytoplasmic integration of iron nanoparticle occured after 72 hrs of incubation. A similar finding was observed with Prussian blue staining in MIRB labelled human MSCs, human cervical carcinoma (HeLa) cells and CG-4 cells (15). The effects of iron oxide–poly (L-lactide) nanoparticles was studied in human BM-MSCs (15); in Cynomolgus monkey BM-MSCs labeled with MIRB (8, 10); in rat BM-MSCs (16); in human neural progenitor cells labeled with MIRB (1, 12).
Viability assay
In the present study, viability of the MIRB labelled oADMSCs ranged between 98-99%, which is in accordance to (17) who worked on sheep BM-MSCs which consisted of both haematopoietic and mesenchymal stem cell population in contrast to adipose tissue which included only the mesenchymal stem cell population. However, in cynomolgus monkey BM-MSCs at MIRB concentrations of up to 30 µg Fe/ml, viability (95–99 %) was not significantly different compared with unlabelled MSCs (98–100 %) (8). For higher labelling concentrations, above 30 µg Fe/ml, viability monotonically decreased and reached a value of 78.8 % at MIRB labelling concentrations of 100 µg Fe/ml.
CONCLUSION
Labelling the oADMSC using MIRB does not affect the viability and proliferation of the cells in-vitro between passages when the contrast agent is used at minimal level (25 µg Fe/ml). The viability is estimated by Trypan blue exclusion test and proliferative capacity is assessed based on the percentage of cell confluence between passages. The present in-vitro study on MRI tracking of MIRB-labelled cells will serve as a model for in-vivo study.
ETHICAL APPROVAL
The present study was carried out after getting approved by the Institutional Ethical Committee for Stem Cell Research and Regenerative Medicine of Tamil Nadu Veterinary and Animal Sciences University.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article