INTRODUCTION
The intensification of the production in poultry farms increases the risk of diseases, expending the use of antimicrobial prophylactic, control or treatment agents (1, 2). Fluoroquinolones which are the most used antimicrobial agents in veterinary medicine are used intensively in poultry production (1, 3).
Marbofloxacin (MAR) is a fluoroquinolone effective against a wide range of important poultry pathogens, including Mycoplasma gallisepticum, Escherichia coli and Pasteurella multocida. It has a low plasma protein binding, it benefits from a rapid and complete absorption, a broad distribution with good concentrations in tissues and bodily fluids, a moderate elimination and a high bioavailability, which are all ideal pharmacokinetic characteristics to use in poultry (4-8).
There are factors that modify this pharmacokinetic behavior such as race, age, weight, environmental factors, among others. Failure to identify these factors leads to errors in predicting the dose-exposure relation within a population (9, 10). It is important to know the modifications that these factors bring about in the pharmacokinetics of drugs in food producing animals, in order to improve therapeutic management and generate food free of pharmacological residues (11).
Finally, the objective of this study is to evaluate the differences in the disposition and pharmacokinetic behavior of MAR in broiler chickens, administered orally and intravenously, in comfort temperature (spring), summer and winter.
MATERIAL AND METHODS
Experimental design
For the study, healthy Ross broiler chickens (n=345) were used. They were 30 days old and each weighed 0.98 ± 0.02 kg. They were randomly divided into 6 groups, a spring group A (n=50) and group B (n=65), a summer group C (n=50) and a group D (n=65), a winter group E (n=50) and a group F (n=65).
For each time of the year, the chickens were placed in a room that maintained the environmental conditions of the study station: spring group with an ambient temperature of 18.6 ± 2.5 °C, winter group of 12 ± 2.9 °C and summer group of 26.6 ± 2.3 °C. The temperature was recorded every 8 hours during the study. The birds received food and water ad libitum.
Chickens were given a single dose of 2 mg/kg of Marbocyl 2% (Laboratorio Vétoquinol S.A., Spain) applied intravenously or orally, for the latter the dose was administrated after 12 hours fasting. In groups A, C, E the blood was sampled between 0.08 to 24 hours, while in groups B, D and F it was between 0.25 to 120 hours.
Sample processing
Five chickens were used for each sample extraction time. Blood collection (5 ml) was performed in the axillary vein, and the blood was placed in heparinized tubes and immediately centrifuged for 10 minutes at 2,500 rpm, to obtain the plasma for the study.
To extract the analyte (MAR), a liquid-liquid extraction was used. 200μl of plasma was placed in an eppendorf tube, 800μl of methanol, 200μl of deionized water and 40μl of enrofloxacin (2.5 μg/ml) were added as an internal standard. The determination was performed by high performance liquid chromatography (HPLC, Hewlett-Packard 1050 equipment), with a fluorescence detector (Hewlett-Packard 1046-A) and C-18 column (Octadecylsilane 5μm, 250 cm x 4.6 mm). Analytes were eluted isocratically with a flow rate of 0.8 ml/min, using a mobile phase consisting of deionized water, acetonitrile, and triethylamine (790:200:10 v/v/v) (12, 13).
Peak areas in chromatograms of the samples were quantitated using the internal-standard technique, by use of solutions of marbofloxacin and reference standard (enrofloxacin). Standard curves were linear (r2 > 0.99) for concentrations ranging from 0.039 to 1.25 μg/ml of plasma for marbofloxacin, and quantification limit was 0.006 μg/ml and the relative recovery was 86.2 ± 3.75% for the ranges 0.0024 - 2.5 μg/ml (12).
Pharmacokinetic analysis
The pharmacokinetic study was performed using the Non-compartmental PK Solution 2.0 pharmacokinetic program (14), also using the average MAR concentrations obtained by sampling time, the way of administration, and the average weight of each study group. In addition, marbofloxacin absolute bioavailability (F) for oral administration was calculated by comparing areas under the intravenous plasma curve (AUCiv) with the oral plasma curve (AUCpo) (15).
Statistical analysis was performed with the software InfoStat (16) ANOVA test and Tukey’s test to compare plasma concentrations and MAR pharmacokinetic parameters between spring (control), summer and winter.
RESULTS
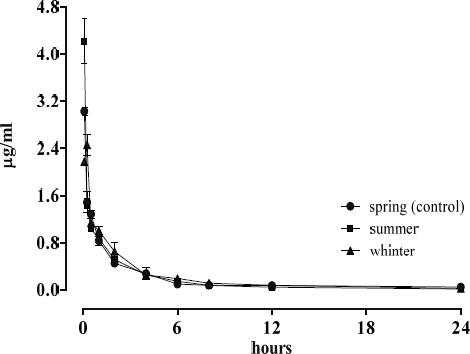
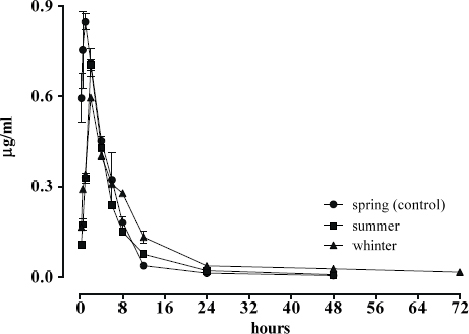
Fig. 1 shows the mean plasma concentrations of MAR in broilers in different seasons by intravenous route and Fig. 2 by oral administration.
Figure 1. Plasma disposition curve concentrations of MAR (μg/ml) after intravenous administration in chickens (2mg/kg) in different seasons
Figure 2. Plasma disposition curve concentrations of MAR (μg/ml) after oral administration in chickens (2mg/kg) in different seasons
MAR concentration by oral application (Fig. 2) in winter is significantly lower (p<0.05) than control (spring), with a reduction in total concentration of 46 ± 4.1 %. No statistically significant differences were observed with the intravenous administration between spring and the other two seasons (p>0.05).
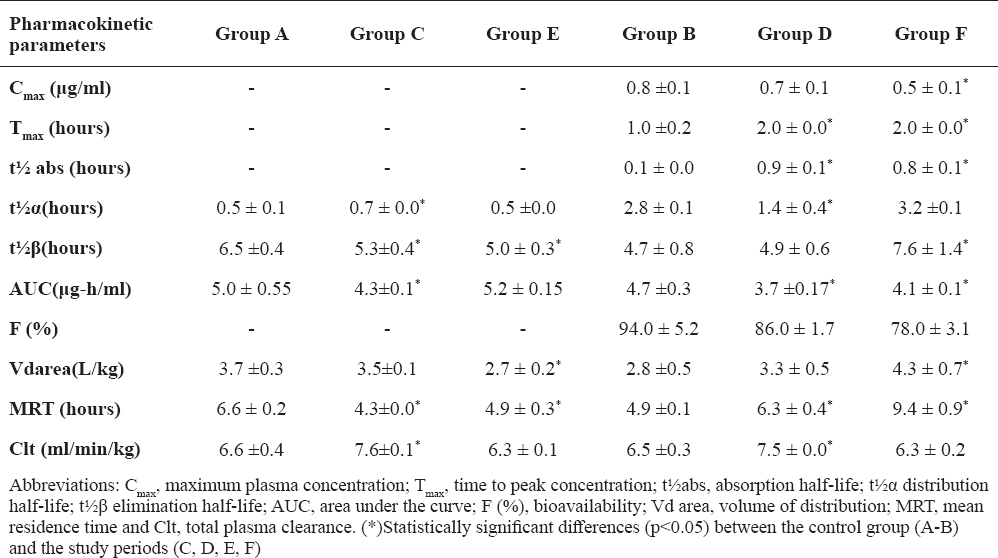
In Table 1, Cmax is lower 39 ± 5.1 % (p <0.05) in winter than in the control period (spring). MAR has a significantly slower and lower oral absorption (p<0.05) in winter and summer compared to spring, which is consistent with the Tmax of 2 hours.
Table 1. MAR pharmacokinetic parameters (mean±sd) after intravenous and oral 2 mg/kg single dose in broiler chickens, at spring (A-B), summer (C-D) and winter (D-F)
Table 1 shows that the elimination of marbofloxacin depends on the season the bird has been exposed to. In winter with the oral administration (group F) there is an increase in the elimination half-life (7.6 ± 1.4 hours), coinciding with the increase of the MRT (9.4 ± 0.9 hours), determining a slower elimination and greater permanence of MAR in the chickens in winter compared to spring and summer (p<0.05). On the other hand, in summer by oral application of MAR (group D), it has a decreased elimination half-life (4.9 ± 0.6 hours), which coincides with the short MRT of 6.3 ± 0.4 hours.
In summer the total plasma clearance (7.6 ± 0.1 ml/min/kg) is significantly higher (p<0.05) compared to the other two seasons. Oral bioavailability of MAR in 86 ± 1.7% and 78 ± 3.1% winter chickens was observed, decreased compared to the spring of 94 ± 5.2 %.
DISCUSSION
The plasma pharmacokinetic behavior of intravenous marbofloxacin at each season of the year is similar to the one described by other authors (4, 6, 7), with a high oral bioavailability which demonstrates that marbofloxacin maintains rapid dissolution in the intestinal environment characteristic of fluoroquinolones (17). In winter, the detection of MAR in plasma is extended to 72 hours post application.
The rapid oral absorption, wide distribution and moderate elimination of MAR observed in birds in the spring is similar to that obtained by other authors (4, 6, 7). The oral absorption of MAR in summer and winter is slower and smaller compared to spring. A decrease of 15 ± 0.8% and 39 ± 5.1% of Cmax was observed in both summer and winter, coinciding with the decrease in oral bioavailability. This is due to the low hardiness of chickens to ambient temperature variations, with an ideal range between 18 to 22 °C (18). This leads to rapid thermoregulatory physiological responses, such as slowing and diminution of the intestinal and stomach peristaltic movements, without modifying those of the proventriculus (19, 20).
There is evidence that fattening chickens exposed to temperatures of 31°C present alteration in the enterocytes and intestinal mucosa, reducing the absorption capacity (21), which may be due to the decrease in bioavailability of marbofloxacin in summer.
The pharmacokinetic parameters of marbofloxacin: clearance, elimination half-life (t2/1β) and MRT (Table 1) by intravenous and oral application, determine that the elimination of MAR in broiler chickens is faster in summer due to the physiological changes that the organism exerts to non-evaporative heat loss, by increasing water consumption and urine production (19).
The physiological mechanism of thermoregulation in birds is regulated on the one hand by the vasomotor system. In cold situations, peripheral vasoconstriction is present to irrigate the most important organs (brain, liver, lungs, etc.), explaining the greater persistence of MAR in winter (72 hours), whereas in heat situations peripheral vasodilation and increased blood flow velocity are developed, eliminating non-evaporative heat (19, 22), coinciding with the rapid elimination of MAR in summer (48 hours).
The rapid elimination of antibiotics in summer was not observed by Nawaz (23) in sheep for sulphadimidine, rather a slower elimination in summer, assigning this difference to the physiological difference in the thermoregulation of birds and mammals.
Finally, Sun et al. (24) in Paralichthysolivaceus worked with different water temperatures and the pharmacokinetic behavior of difloxacin, concluding like our study that in summer there is a significantly higher rate of drug clearance and decreased half-life elimination, which coincides with the pharmacokinetic results of our study.
CONCLUSION
The conclusion is that in summer and winter there is a decrease in the plasma concentrations of marbofloxacin by oral application in broiler chickens compared to spring, significantly modifying the pharmacokinetic parameters.
It was determined that the therapeutic use of marbofloxacin in broiler chickens varies according to the season of the year, probably due to the physiological thermoregulatory responses of the bird to the ambient temperatures. It is of importance in later studies to see if it modifies the pharmacological residues that can have repercussions in humans as animal producing food. This last point will be investigated in future studies.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article