INTRODUCTION
There is a risk of perioperative mortality related to the anesthesia of small animals. Studies from referrals and university departments have shown a higher risk of mortality compared to the private veterinary practices, due to different characteristics of patients and due to the performing of more complex surgical activities (1). Anesthesia is characterized as a controlled and reversible process. Unfortunately, the primary effect of anesthetics is a depressive action on physiological processes (2). The administration of anesthetics produces a controlled and reversible depression of neurologic cortical activity. Unfortunately, the anesthetics also produce adverse effects such as hypotension, hypothermia, and dose-dependent respiratory depression. As a consequence, anesthetics cause hypoxia and acidosis which affect the recovery from anesthesia and they can have fatal implications for critically ill patients (3). One main objective for the anesthetist is to ensure the supply of oxygen to the patient´s tissues. The distribution of oxygen in the body is the product of cardiac output and hemoglobin oxygen saturation (4). Phosphate is the main component in the formation of 2,3-diphosphoglycerate (2,3 DPG), which is required for normal oxygen release from hemoglobin to the tissues (5). Inadequate supply of oxygen for cellular metabolism can give rise to lactic acidosis (6). Many papers have documented the occurrence of reduced serum calcium concentrations during anesthesia (7, 8, 9, 10), but overall only few studies have focused on serum calcium and phosphorus concentrations in dogs.
The aim of this study was to examine the influence of general anesthesia on the serum levels of total calcium, phosphorus and acid base status in surgical patients and to compare these findings by anesthetic risk as defined by the American Society of Anesthesiologists (ASA).
MATERIAL AND METHODS
This study included 53 dogs admitted to the Small Animal Clinic which were divided according to the classification defined by the American Society of Anesthesiologists (ASA) into 5 categories of surgical risk (ASA I-V) (12) based on the signalment, history of underlying diseases or surgeries and of previous or current medical treatment of symptoms of various illnesses (burden intolerance, cough, diarrhea, vomiting, etc.), based on the body condition score (BCS, grades 1-5) and others (11, 12).
For premedication and sedation Medetomidine (Cepetor KH 1mg/ml, CP-Pharma, Germany) was used in a dose of 10-20 µg/kg intramuscularly, Butorphanol (Butomidor 10mg/ml, Richterpharma, Austria) 0.2-0.4mg/kg or Morphine (Morphin 1% Biotika, BB Pharma, Slovakia) 0.4-0.8mg/kg, and Diazepam (Apaurin 10mg/2ml, Krka, Slovenia) in the dose of 0.3mg/kg. For induction Ketamine (Narketan 10, Vétoquinol, France) was used intravenously in a dose of 4-5mg/kg.
General anesthesia was maintained via inhalation with a mixture of Isoflurane (Forane, Abbott, UK) and the low flow oxygen via an inhalation device Komesaroff Mini-Com (Australia) with vaporizer-incirquit. No dog was ventilated by means of a mechanical ventilator during general anesthesia. When needed, for patients in ASA IV and V we used ventilation with positive pressure manually. Infusion therapy in the perioperative period was provided 0.9% NaCl (5-10ml/kg/hour). For the groups ASA IV and ASA V, the dosing was 60-90 ml/kg/hour. Dosage and route of administration (s.c., i.m., i.v.) of each anesthetic was adjusted according to the individual patient and clinical circumstances. After premedication for analgesia in orthopedic patients we used Morphine and in every other patients Butorphanol. For patients in the ASA IV and ASA V groups, we did not use Medetomidine as a part of the premedication. Blood collection to analyze the concentration of calcium and phosphorus in the blood serum (volume 1ml) was performed from the peripheral veins – v. cephalica antebrachii, v. saphena lateralis. Blood serum was obtained after centrifugation of clotted blood for 10 minutes at 3500rpm. Blood collection was performed preoperatively before administration of sedatives and anesthetics (T0), under general anesthesia during the surgery in the 30th minute (T1) and 120 minutes after the end of surgery (T2). Evaluated parameters were measured with automated analyzator Cobas c111 Roche (Switzerland) using diagnostic tests Roche (Switzerland). The physiological range values defined for calcium were 2.05-2.86mmol/l and for phosphorus 0.90-1.91mmol/l (13). Blood for acid base analysis was collected anaerobically into the capillary tube (volume 200µl) containing heparin lithium and analyzed immediately after collection without storing samples with blood gas analyzer (Easy Medica Blood Gas, GMI, USA). Physiological pH values of venous blood were set at 7.32-7.40 (14).
Data was processed and analyzed according to the evaluated end points, groups of animals and time periods. The results were processed by stating mean values and standard deviations (SD). The significance of changes during individual perioperative periods was analyzed with Friedman (F) non-parametric test. Significance of differences between groups of dogs (ASA I to ASA V) was analyzed with Kruskal-Wallis (K-W) non-parametric test. In both cases, we used Dunn´s comparative post-test. Statistical analysis was performed using statistical software GraphPad Prism version 3.0. Results were considered statistically significant if P < 0.05.
RESULTS
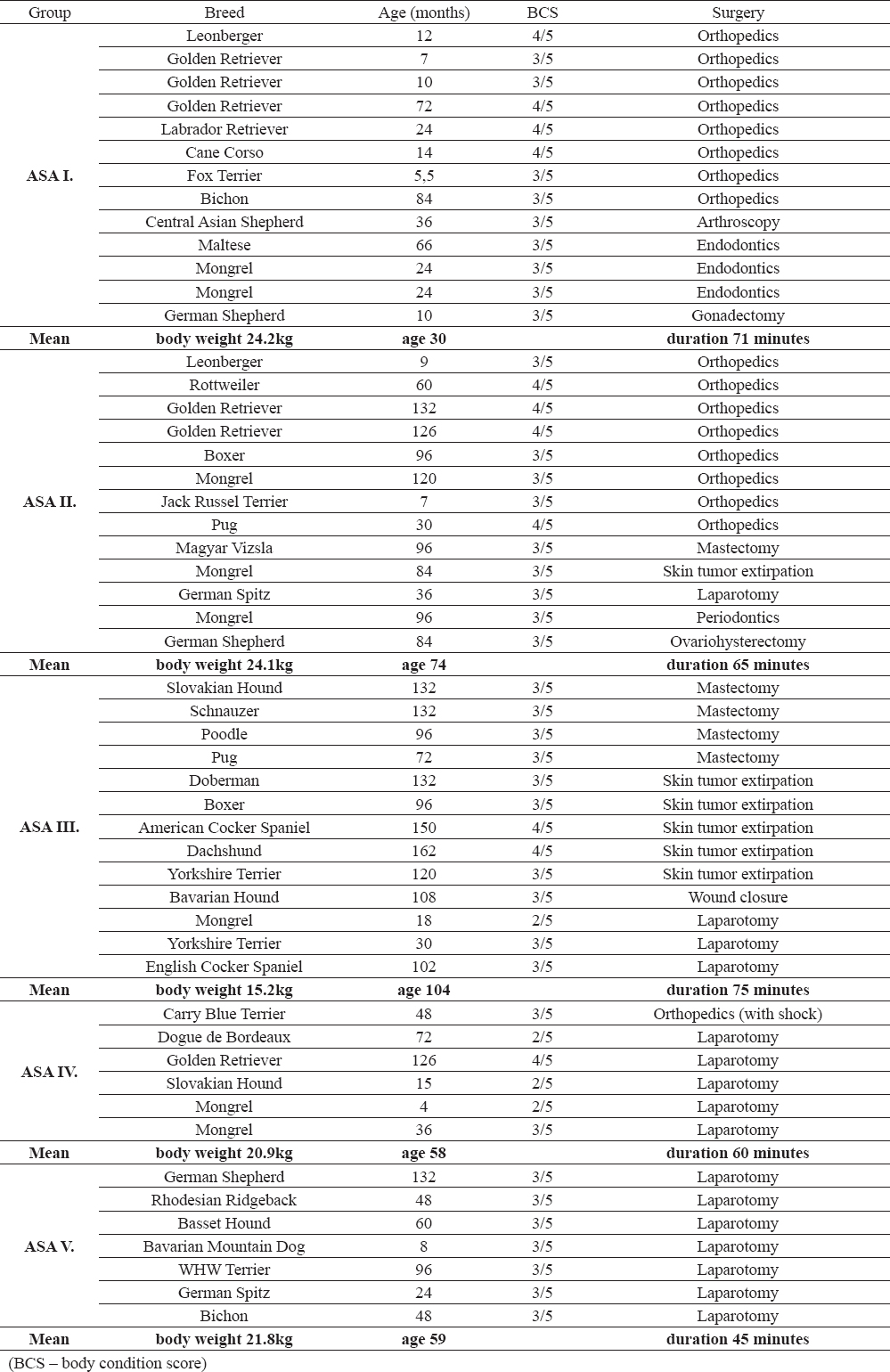
The group ASA I consisted of 13 patients (8 females and 5 males). This group contained surgical patients not showing any signs of internal diseases and which were in a good physical condition. This group of dogs was set as a control group. The average duration of the surgical procedure – the anesthetic time for this group was 71 minutes. Group ASA II included 13 patients (7 females and 6 males). The average duration of the surgical procedure was 65 minutes. The ASA III group comprised of 13 patients (10 females and 3 males) and the average duration was 75 minutes. In the ASA IV group there were 7 patients (3 females and 4 males) affected with serious medical conditions. The average duration of the surgical procedure was 60 minutes. In the ASA V group there were 7 polytraumatic patients (5 females, 2 males) and the average duration of the surgical procedure was 45 minutes. Detailed description of patients and operative procedures is given in Table 1.
Table 1. Preoperative distribution of dogs in the ASA groups and their characteristics
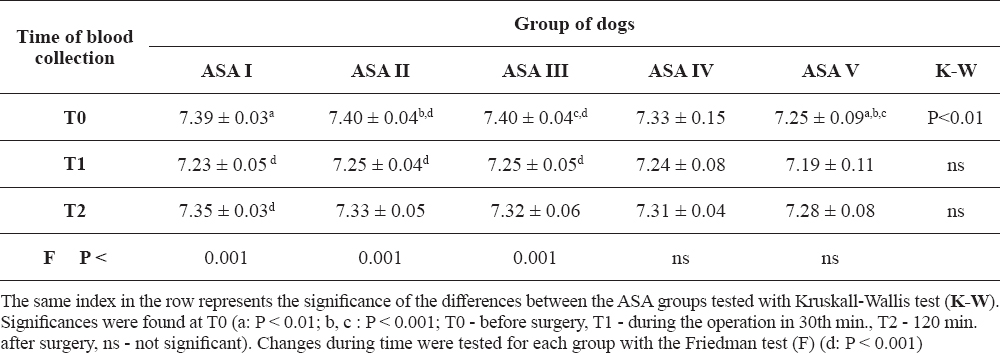
The venous blood pH during the preoperative period (T0) was under the physiological values only in the ASA V group of dogs (Table 2). Significant changes in the pH levels during the reporting period were recorded in the ASA I, ASA II, and ASA III group (P < 0.001). Venous pH was significantly lower in ASA V as compared to ASA I, ASA II and ASA III. The lowest average values of pH were found in all groups during the surgical procedure (at the 30th minute). 120 minutes after the surgical procedure, the rise of blood pH was seen in all groups except for the ASA V.
Table 2. Blood pH levels in the ASA groups of dogs in perioperative period (mean ± SD)
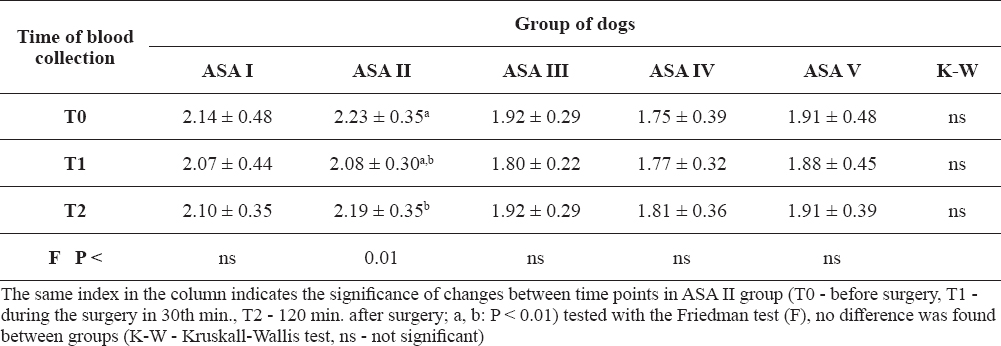
The pre-operative measurements revealed that the average concentration of calcium in the blood serum was below the physiological range in the groups ASA III, IV and V (Table 3). Most dogs with hypocalcemia during the whole period were in the ASA III group (69.2%). After induction of general anesthesia (in the 30th minute), a decrease in the concentration of calcium was observed in all groups, except the ASA IV group. Changes in the concentration of calcium were significant in the ASA II group of dogs (P < 0.01), while mean values after induction of general anesthesia were within the normal range.
Table 3. Measured calcium concentrations in blood serum (mean ± SD)
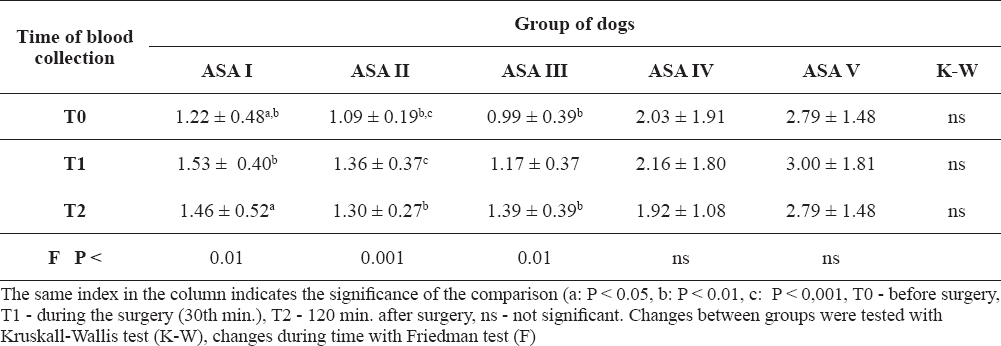
The average concentrations of phosphorus in the serum of all groups of dogs was in the physiological range preoperatively. The lowest average value was recorded in the ASA III group of dogs and the highest in the ASAV group of dogs (Table 4). Changes in phosphorus concentrations during the monitored period were significant in groups ASA I (P < 0.01), ASA II (P < 0.001), and ASA III (P < 0.01). No significant differences in the serum phosphorus level were found between the groups at any of the monitored time periods.
Table 4. Measured phosphorus concentrations in the blood serum (mean ± SD)
DISCUSSION
After admission of dogs to the clinic, history collection and clinical examination was performed for each dog. Medetomidine (except for groups ASA IV and V) and Butorphanol were administered, which are known to cause significant, dose-related increase of PaCO2 and a decrease of PaO2 and the pH values of blood (15, 16, 17). This fact could partially affect the results of our study. In the available literature there are only a few studies dealing with effects of anesthesia and surgical procedures on the concentration of calcium and phosphorus in animals. Much of the information is taken from human medicine and use the ionized calcium level. In the study conducted by Aguilera and Vaughan in 2000 (10) it was found that the value of ionized calcium is affected by changes in blood pH. Low levels of serum calcium below the physiological margins were recorded preoperatively in the ASA III, IV, and V groups in our study. After administration of anesthetics the concentration dropped even more, which may present a potential risk factor. The ASA III group of dogs consisted mostly of oncological patients and this group is characterized by the highest average age of dogs. In general, the occurrence of hypercalcemia is reported in patients at the time point when the neoplastic process is already apparent and it may occur in approximately 10% of malignant cancer patients according to literature (18). In our group of cancer patients hypocalcaemia was measured, which worsened during the surgical procedure. Intraoperative or postoperative hypocalcemia has been previously observed by several authors and was attributed to inhalation anesthesia in humans, dogs and horses (7, 19, 20, 21). This decrease in calcium concentration could be caused by the premedication or induction of anesthesia. In studies when different anesthetic protocols were used including opioids, benzodiazepines, ketamine, and isoflurane (22) there was a significant reduction in arterial and venous concentrations of ionized calcium in healthy dogs during general anesthesia lasting less than three and a half hours. In addition, acidosis and hypocalcaemia commonly occur in critically ill dogs (23, 24, 25) and according to some authors also during the general anesthesia (8, 26, 27).
In this study we recorded an increase in phosphorus concentrations in all groups of dogs after administration of anesthetics. A significant rise in the phosphorus was observed in the ASA I, II, and III groups of dogs. Changes in plasma concentration of phosphorus during anesthesia with ketamine and diazepam have been previously observed in 2003 by Gonzáles-Gil et al. (28). Later in 2010 Gonzáles-Gil et al. (29) published a study on healthy rabbits where after administration of isoflurane there was a reduction in serum calcium and an increase in phosphorus concentrations, which is what was observed in our study. Shiber and Mattu in 2002 (5) have suggested that hyperphosphatemia may be caused by hypomagnesemia, hypernatremia and metabolic acidosis. Hyperphosphatemia could be also due to young age of patients (30, 31) which is true for the first group of dogs (ASA I) in our study.
In the case of dropped phosphorus concentration (32), the authors did not conclusively state that hypophosphatemia may be indicative of an emerging sepsis. The groups in our study did not include any patients with sepsis. In one dog in the ASA I group alkalosis and hypophosphatemia occurred in the preoperative period.
This study has several limitations, namely the small and different numbers of patients in each group and the fact that there was no measurement of ionized calcium. These results are indicative and further prospective studies are needed to verify the full impact of the severity of condition.
CONCLUSION
Following the administration of sedatives and anesthetics, acidaemia occurred in all dogs and significant changes in the serum calcium levels were observed. The lowest levels of calcium during the whole perioperative period were detected in patients with skin and mammary cancers, although no patient suffered from serious hypercalcemia or hypocalcemia. Influence of anesthesia led to a significant increase in the concentration of phosphorus, but the values remained within the physiological range. Further prospective studies are needed to verify the full impact of the severity of these conditions.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article