INTRODUCTION
Rattus rattus (Rodentia: Muridae), also known as the black rat, is one of the most common harmful rodents in both hemispheres of the planet. As a typical omnivorous mammal, it often lives near inhabited buildings, around landfills and sewers. An increasing density of rat and mouse populations is due to their high reproduction ability, rapid adaptability to changing environmental conditions and the opportunity to select resistance to some rodenticide substances (1). It has been confirmed that these harmful rodents play an important role as a reservoir of many pathogenic agents transmissible to humans and pose a serious threat to the public health. (2). Rat helminth fauna includes a variety of species such as cestodes Hymenolepis diminuta, Hymenolepis nana, Taenia taeniaeformis larval stage (Strobilocercus fasciolaris) and nematodes Trichuris muris, Syphacia spp., Capillaria hepatica and Aspiculuris tetraptera (2-7). Infection by A. tetraptera, Hymenolepis spp. and Oxyuridae spp. in rats and mice in Bulgaria has been reported (8). The rodents also play an important role as an intermediate host and a source of Echinococcus multilocularis infection for carnivorous (9) and as a reservoir of Trichinella spiralis for pigs and humans (10).
Generally, the synantropic rodents are an important factor in human parasitic diseases. Due to the lack of updated information on the problem our aim was to investigate the helminth fauna of the black rat in some regions of Stara Zagora district.
MATERIAL AND METHODS
The study was carried out in four rabbit farms and a zoo near Stara Zagora. It included examining carcasses of 67 black rats collected after a scheduled exterminating by placing the poisoned bait chunks containing 0.005% bromadiolone. Deratisation in the studied sites usually takes place several times a year depending on the density of rodent population.
Rat identification was achieved by measuring the significant somatometric and craniometric parameters described by Peshev et al. (11). After necropsy, the gastro-intestinal tracts and all organs within the abdominal and thoracic cavities were inspected for presence of adult helminths or larvae. The intestinal content was examined by standard salt flotation and sedimentation techniques for detection of parasite eggs. Liver smears were prepared to observe the eggs of C. hepatica. Pieces of diaphragm and masseters were examined by compression method to detect muscle Trichinellalarvae. Adult worms and eggs were identified by using the descriptions of Baker (12).
RESULTS
Single and mixed infections by up to three helminths were found in 53 (79.1%) of all the examined rats. Eight species were identified: four belonging to Class Cestoda (H. diminuta, H. nana, T. taeniaeformis larvae, T. polyacantha larvae), two to Class Secernentea (S. obvelata, A. tetraptera) and two to Class Adenophorea (C. hepatica, T. muris).
Out of all the investigated rats, 27 (40.3%) were infected by one helminth species and 26 (38.8%) by more than one. Mixed infections with two and three helminths were found in 32.8% and 6.0%, respectively. Hymenolepis diminuta was a predominant species in cases of single infections (23.9%). Hymenolepis nana was the main agent in mixed infections (28.4%). The most frequent co-infection was H. nana/ H. diminuta (16.4%).
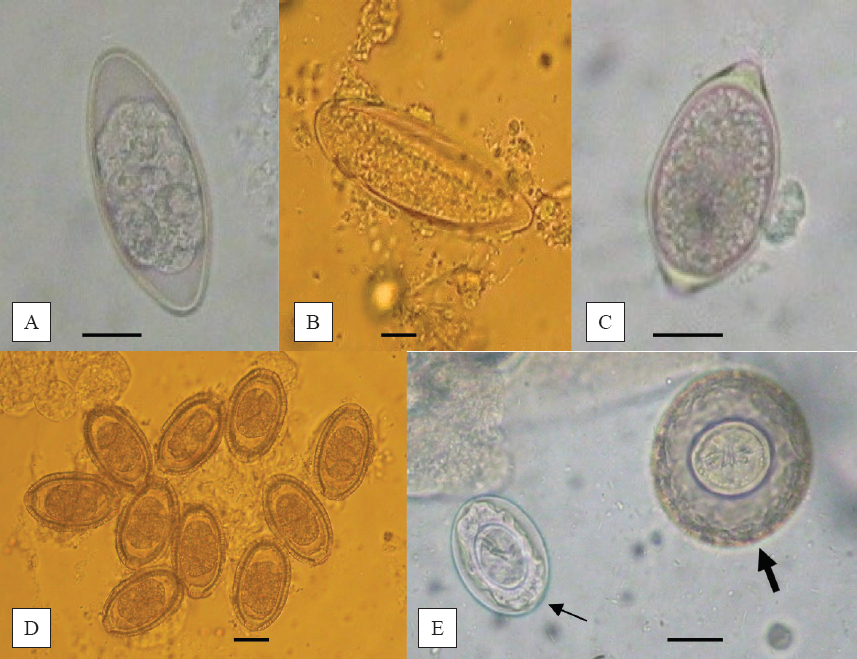
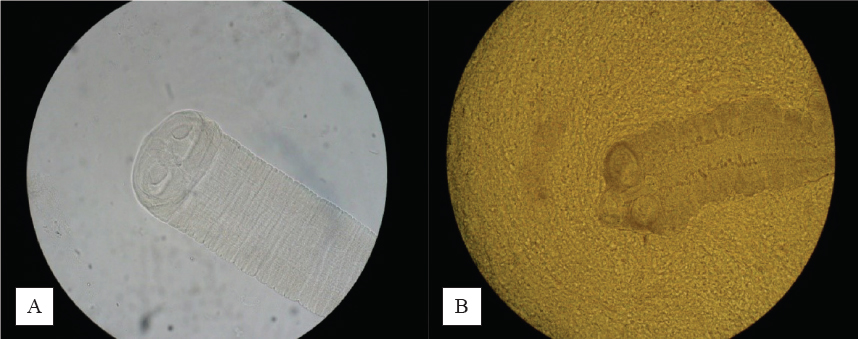
No trematodes and acanthocephalans were discovered. Muscle Trichinella larvae and cysts of E. multilocularis were also not detected. All additional results are presented in Tables 1 and 2. Detected eggs, adult helminths and larvae are shown in Fig. 1, 2 and 3.
Table 1. Frequency of helminths in black rats (n = 67)
Table 2. Frequency of single and mixed infection in black rats (n=67)
Figure 1. Eggs of A. tetraptera (A), S. obvelata (B), T. muris (C), C. hepatica (D), H. diminuta (thick arrow) and H. nana (thin arrow) (E) (magnification bar 20 μm)
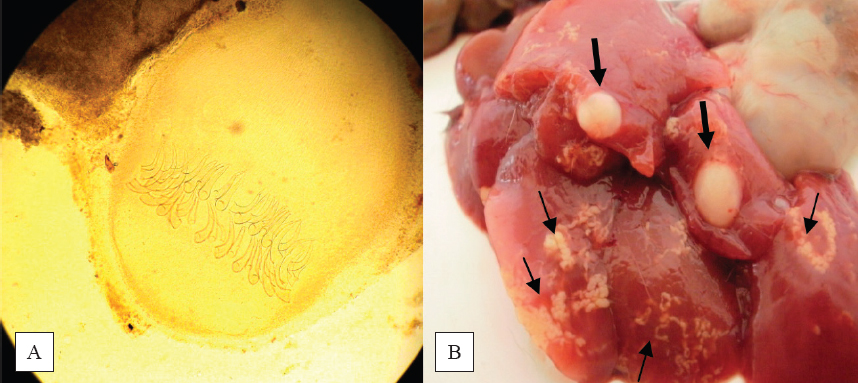
Figure 2. Scolices of H. diminuta (A) and H. nana (B) (40X)
Figure 3. Metacestode of T. polyacantha (A) (40X), T. taeniaeformis larvae (thick arrows) and C. hepatica migration routes (thin arrows) (B)
DISCUSSION
Reports from Serbia (1) and Croatia (7) showed prevalence of hymenolepidids among rodents in 30.5% and 36.9% of cases, respectively, which fully corresponds to our data. In contrast, those infections in humans are rare. According to Genov (13) the incidence of H. diminuta infection in humans is sporadic and varies between 0.001% to 5.5% of the human population (14).
In the present study we found high prevalence of H. nana infection which is in agreement with the data presented by Gilioli et al. (15). The authors detected H. nana in 53.3% of examined rats. The possibility of direct H. nana transmission between humans and rodents without the presence of an intermediate host (16) and autoinfection possibility (17, 18) explains the high prevalence of this cestode in humans compared to H. diminuta infection (16). In addition, H. nana is found mostly in countries with tropical and subtropical climates and affects about 21 million people (19). In Bulgaria as a country with a typical temperate climate, Genov (13) reported H. nana infection in 0.9% of the human population. A new data presented by Kurdova et al. (20) revealed 116 cases (0.023%) in 2010.
Our results showed a low prevalence of C. hepatica. Liver smears revealed migration routes filled with eggs of Capillaria in 9.0% of rats, which is not in accordance with some reports (21, 22, 23). The authors reported the prevalence of hepatic capillariasis between 23% and 75%, but in brown rat (Rattus norvegicus). In contrast, hepatic capillariasis is a rare condition in humans (24). The transmission of C. hepatica among animals and humans occurs by ingestion of embryonated eggs which are released into the environment after the host death (25). In the surveyed buildings included in this study, extermination with apparent effect is regularly done, followed by collection and destruction of dead rodents. This approach has proven very effective against the distribution of both capillariasis and echinococcosis due to E. multilocularis. Such is the case with feline T. taeniaeformis. Its larvae develop in murid rodents which suggests a high prevalence among rats and mice, generally. In our investigation, the access of stray cats into animal buildings is limited. Larvae of T. taeniaeformis among population of R. rattus was found to vary between 3.5% and 4.34% (26, 27).
A lower infection rate by pinworms (A. tetraptera and S. obvelata), whipworm (T. muris) and larvae of T. polyacantha was recorded in this study. Our results also showed almost equal number of rats with a single (n=27) and mixed infection (n=26). The combination between H. nana and H. diminuta was the most common. In Bulgaria, Tsankova et al. (28) investigated helminth fauna of black rat living in food industry enterprises. The authors revealed S. obvelata, H. nana and H. diminuta.
CONCLUSION
The presence of the black rat around animal facilities poses a high risks not only for animals, but also for farm teams. Results of our study showed that more than half of examinated rats (79.1%) were infected with one (40.3%) or more helminths (38.8%). Hymenolepis diminuta and Hymenolepis nana were the most prevalent species in parasitocenosis. Moreover, the humans are able to be infected by H. nana. Based on these findings, we believe that it is necessary to perform a systematic control aimed at full rat eradication in order to prevent zooanthroponoses.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.