INTRODUCTION
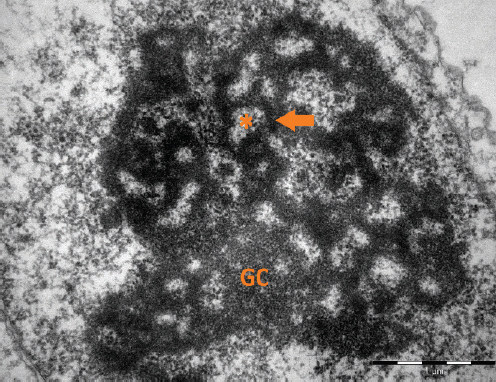
The main goal of our review is to offer a compact view on nucleolus and all its forms in consideration of current knowledge and to suggest the best way of its application in the area of reproduction biology which can lead to an improvement of the development potential of early embryos used in veterinary or human medicine. The nucleolus is the intranuclear structure (organelle) in eukaryotic cells, usually located close to the nuclear membrane. It is a multifunctional organelle that is involved in rRNA synthesis and its posttranscriptional modifications, formation of ribosomes, cell cycle regulation, proliferation, chromosome segregation, reprogramming, common stress reaction, nuclear transport and many other functions. Interestingly, nucleoli in oocytes have several forms and functions, which vary during oocyte growth and early embryonic development. The typical differentiated nucleolus consists of fibrillar centers (FCs), dense fibrillar components (DFCs) and granular components (GCs) and can be found in somatic cells, stem cells, growing oocytes and in cells of advanced developing embryos (Fig. 1a).
Figure 1a. An electronogram of nucleolar organization in somatic cell. The three nucleolar components are visible: the fibrillar centres (asterisks), the dense fibrillar component (arrow), and granular component (GC). Scale bar: 1.0 μm
NUCLEOLI IN OOCYTES
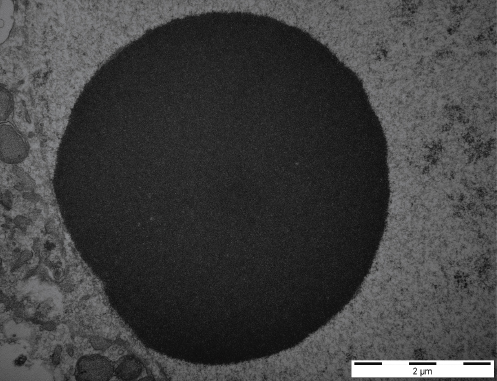
Very profound morphological and functional changes can be seen in nucleoli during oocyte growth. When the oocyte grows, the nucleolus has similar structure when compared to somatic cells, i.e. contains FC, DFC and GC with intensive RNA synthesis (1). By contrast, in fully grown oocytes the nucleolar structure differs completely. The nucleoli of fully grown oocytes are visible as dense compact structures composed almost exclusively of fibrillar material and are called as nucleolus-like bodies (NLBs) (Fig. 1b) (2).
Figure 1b. An electronogram of NLB in fully grown porcine oocyte. Scale bar: 2.0 μm
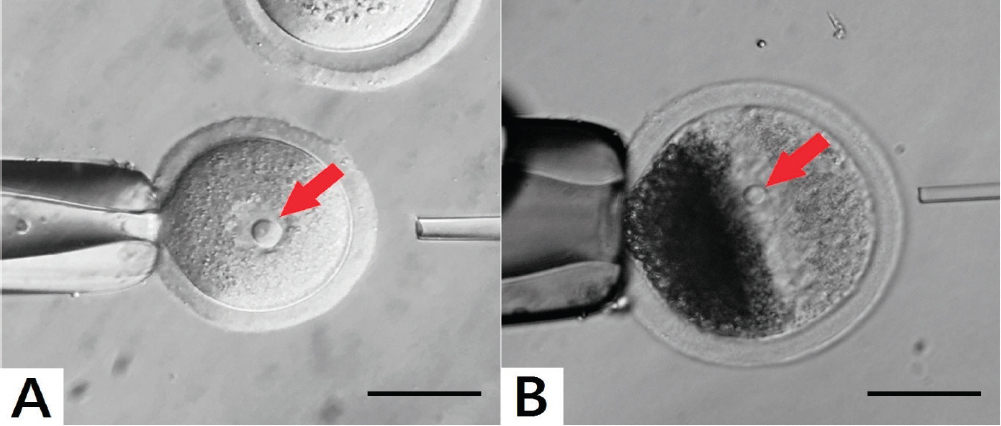
In mouse and human oocytes, they are visible with conventional light microscopy (Fig. 2). Accordingly, RNA synthesis decreases in nucleoli of this oocyte developmental stage (3). The fully grown oocytes do not synthesize ribosomal RNA, but synthesis of the heterogeneous nuclear RNA continues until the resumption of meiosis (4).
Figure 2. Visualisation of NLB in germinal vesicles (GV – red arrow). (A): Mouse oocyte. (B): As porcine oocytes contain many dark lipid particles they must be centrifuged in order to visualize GV with NLBs. The images were produced by the Olympus IX71 inverted microscope. The sample A was originated from mouse large antral follicle of CD – 1 IGS and the sample B was originated from porcine large antral follicle. Scale bar: 50.0 μm
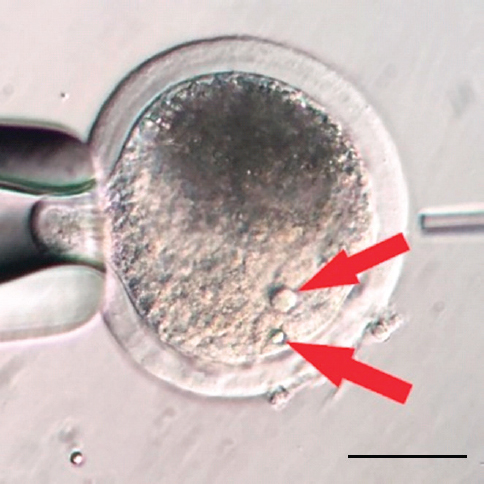
Oocytes usually contain a single NLB within the nucleus, however, sometimes more than one NLB can be seen (Fig. 3). It is not known, if these oocytes have higher or lower competence to mature or develop after fertilization, but according to our preliminary studies they are not suitable for enucleolation by micromanipulation. It has been commonly accepted that oocyte and zygote NLB/NPB serve as repository of material from which differentiated nucleoli in embryos are gradually formed during embryogenesis (5, 6). Recent observations have challenged this developmental biology dogma and showed that they are important only during a very short time period after fertilization (7, 8).
Figure 3. Two NLBs in GV stage of porcine oocyte. The image was produced by the Olympus IX71 inverted microscope. The sample was originated from porcine large antral follicle. Scale bar: 50.0 μm
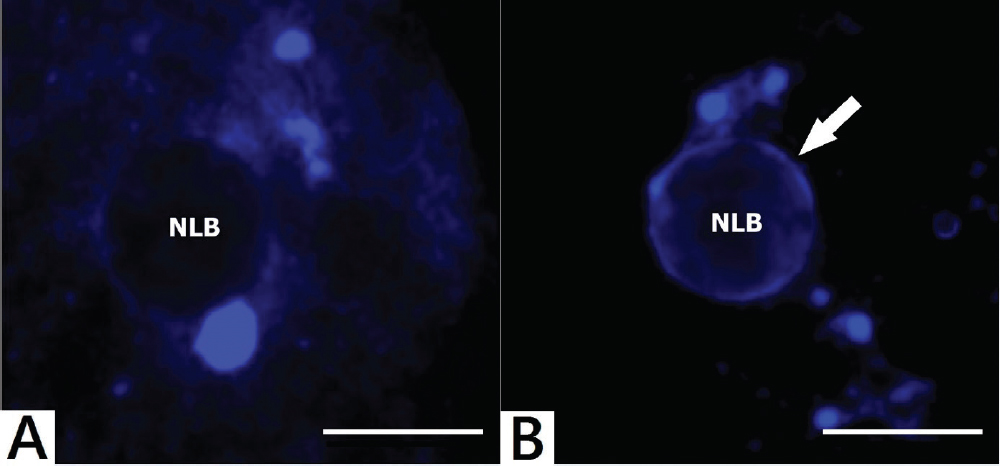
During the oocyte growth, profound chromatin and nucleolus modifications can be observed. In growing oocytes chromatin is dispersed throughout the germinal vesicle (GV) with no close association with the nucleolus (non-surrounded nucleolus; NSN). On the other hand, in fully grown oocytes more intimate association between the NLB and chromatin can be seen (9). The chromatin in fully grown oocytes is in close contact with the NLB and forms the ring or the horseshoe around it – this is called the surrounded nucleolus. (SN) (Fig. 4) (10). Oocytes without close association with heterochromatin can be fertilized or parthenogenetically activated, even they form pronuclei but reach only the two-cell stage (11).
Figure 4. Distinct NLB and chromatin association in mouse oocytes. (A): NSN dispersed chromatin organisation. (B): SN chromatin organisation (chromatin – white arrow). The images were produced by the Olympus BX61 confocal fully automated microscope. Fluorescent staining of chromatin was made by Hoechst 33342 (Sigma 14533). All samples were originated from mouse large antral follicles of CD – 1 IGS. Scale bar: 10.0 μm
It has been shown, that a single mouse NLB isolated from a fully grown oocyte contains approximately 1.6 ± 0.3 ng of proteins (12). However, very little is known about the proteomic composition of NLBs.
During the oocyte maturation, NLB dissolves concomitantly with germinal vesicle breakdown (GVBD) (13).
NUCLEOLI IN ZYGOTES AND EARLY CLEAVAGE-STAGE EMBRYOS
In comparison to immature oocytes at the GV stage, the zygotes typically contain several nucleoli in each pronucleus. During pronuclear development, these nucleoli fuse and form a single prominent structure (8). As it has been commonly accepted until recently that this structure is gradually transformed during subsequent embryonic development into typical tripartite nucleoli, it was also termed as so called “nucleolus precursor bodies – NPBs”.
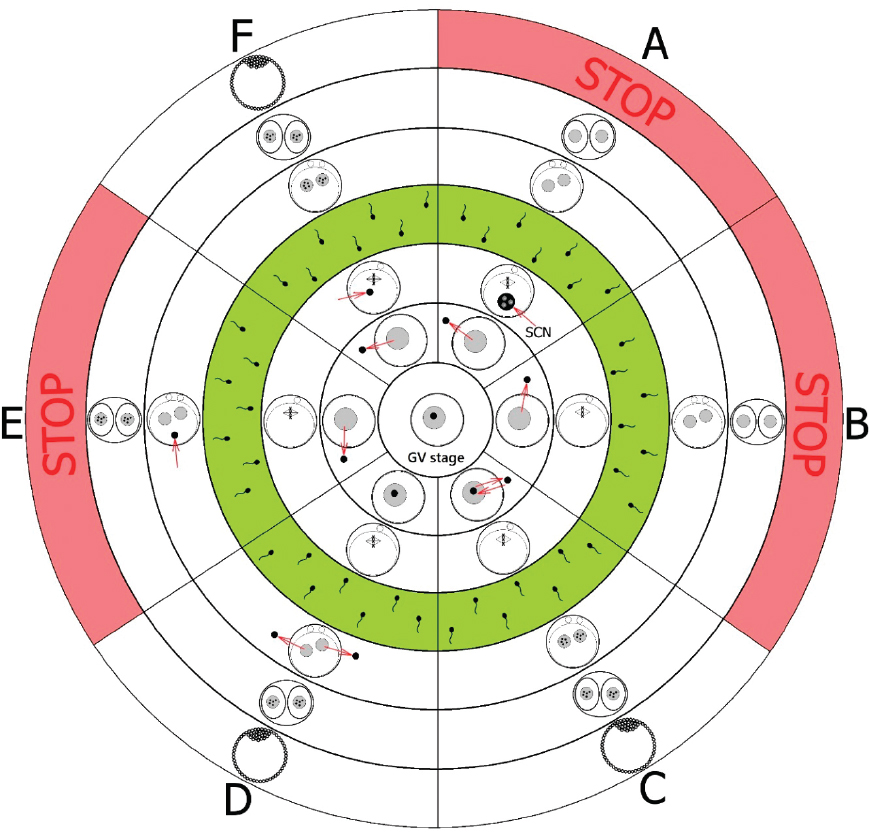
NPBs in zygotes and early cleavage-stage embryos are strictly of maternal origin, they are not formed de novo and they are essential for chromatin remodelling shortly post oocyte fertilization or parthenogenetic activation (14). Accordingly, they are not involved in embryonic ribosome production. Important results obtained by NLB and NPB transfer are summarized in the Figure 5 (1).
Figure 5. Scheme of NLB transfer (nucleolotransfer). SCN – somatic cell nucleus with its nucleolus; Green circle – IVF or parthenogenetic activation; STOP – no development. (A): If NLBs were replaced by somatic cell nucleoli (SCN), the embryo development was stopped and no NPBs in pronuclei were found after parthenogenetic activation or IVF (14, 20, 21). (B): Oocytes from which NLBs were removed have matured to metaphase II, however after parthenogenetic activation or IVF, no NPBs in pronuclei were found and the embryo development was stopped at the two-cell stage (14). (C): If NLBs were re-injected immediately after the enucleolation, these oocytes matured to metaphase II and after parthenogenetic activation or IVF, NPBs in pronuclei were formed and embryos developed to full term (14). (D): When NPBs were removed from late zygotes, no NPBs were detected in pronuclei or nuclei of enucleolated zygotes or in two-cell embryos. However, later (approximately at the four-cell stage) these embryos formed nucleoli de novo and the embryos have developed into the blastocyst stage after parthenogenetic activation or IVF (8). (E): If NLBs were removed from oocytes and introduced back into more advanced parthenogenetic or IVF embryos (originated from enucleolated oocytes, i.e. more than 8 hrs post activation or IVF), the embryo development was stopped at the two-cell stage (22). (F): If NLBs were removed from oocytes and introduced back into less advanced embryos (without NPBs) post parthenogenetic activation or IVF (i.e. less than 7 hrs), NPBs in pronuclei were seen and embryos developed to full term (14, 22)
The NPB in zygotes or in early cleavage-stage embryos differ morphologically from nucleoli in more advanced embryos, stem cells and somatic cells (1). However, in many respects they are very similar to oocyte NLBs because they have dense fibrillar morphology. On the other hand, reticulated nucleoli in somatic cells and advanced embryo developmental stages have typical fibrillo-granular composition (15, 16, 17).
At the two cell stage, mouse embryos become transcriptionally active and begin to synthesize rRNA (18, 19). Throughout this process, NPBs are gradually transformed into reticulated nucleoli (8).
NUCLEOLI IN DIFFERENTIATED CELLS
Typical differentiated nucleolus in somatic cells is an intranuclear organelle consisting of FC, FCS and GC (Fig. 1a) (23). The main function of these nucleoli is the participation on synthesis of ribosomal RNAs (rRNAs), except for 5S RNA. This synthesis is catalysed by RNA polymerase I, primarily located in the nucleolus (13). Other functions of these nucleoli include the control of cell cycle progression, genome maintenance, differentiation and regulation of DNA damage responses differentiation, etc. (1). rRNA is a major structural and functional component of ribosomes. It is created by transcription of rDNA. The cell contains four types of rRNA, which are 120 – 5000 base pairs length and present 80 % of all RNA (24).
During the early embryonic development, NLB changes its fibrillar composition into a tripartite one. This process is associated with the resumption of rRNA synthesis (1).
NUCLEOLOTRANSFER AND ITS APPLICATION
Whilst the somatic cell nucleoli were analysed in detail, very little is known about the composition of NLBs (NPBs). This is a consequence of difficult isolation of this structure from GV oocytes (or zygotes). This has been solved when Fulka, Jr. et al. invented the method of microsurgical removal of NLB from fully grown oocytes called “the enucleolation” (3).
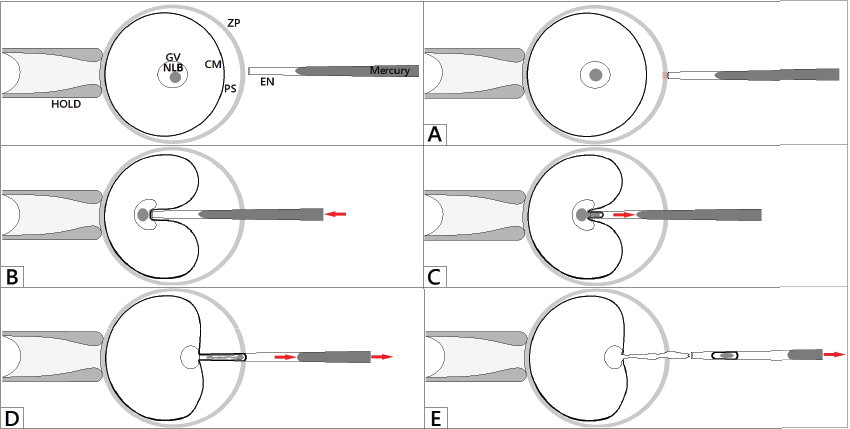
Shortly after the enucleolation, the volume of isolated NLB rapidly decreases and NLBs dissolve in the medium. However, if NLBs are removed from GVs as so called “nucleoloplasts” (i.e. NLB is enclosed with a small volume of the cytoplasm and cytoplasmic membrane), NLB is protected and does not dissolve. All steps of the procedure are described in detail in the Figure 6. It is very important to note, that the isolated NLB is not enclosed with a residual DNA. This was verified by labelling the nucleoloplasts with anti-trimethyl H4/K20 antibody or staining by Hoechst 33342 (12).
Figure 6. Process of enucleolation. HOLD – holding pipette, EN – enucleolation pipette, ZP – zona pellucida, PS – perivitelline space, CM – cytoplasmic membrane, GV – germinal vesicle, NLB. (A): Oocyte is fixed in the stable position by applying the suction in holding pipette. The enucleolation pipette is positioned close to the NLB and penetrates through the zona pellucida. (B): The enucleolation pipette aspirates the NLB, but the cytoplasmic membrane is still intact. (C): The NLB is slowly and gently aspirated into the enucleolation pipette. (D): The enucleolation pipette is then slowly withdrawn from the oocyte. (E): As soon as the enucleolation pipette is outside, additional suction is applied and the NLB slowly penetrates the germinal vesicle like a sand in a sand-glass
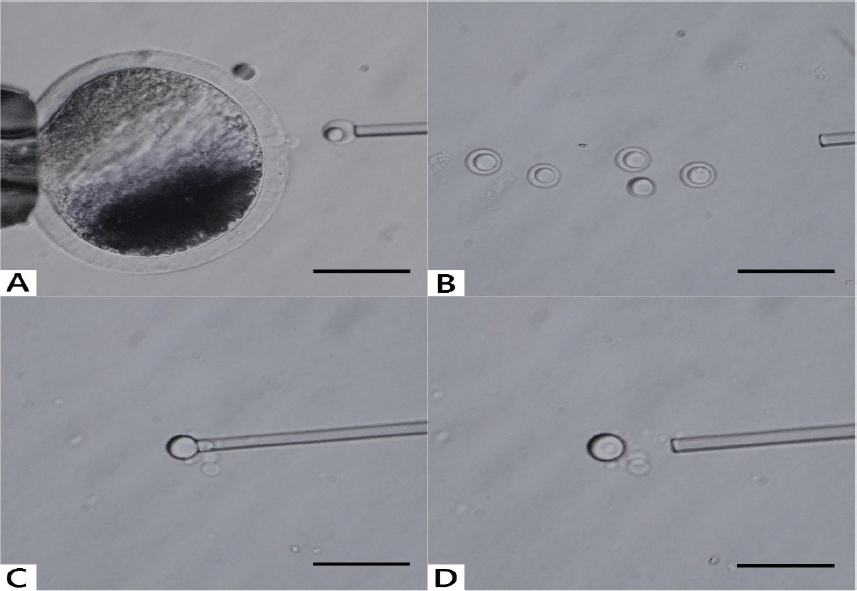
As mentioned above, the collection of sufficient number of NLBs which can be collected in one experiment is rather complicated, i.e. the enucleolation needs certain skills, NLBs can be very easily lost due to their small diameter or if not collected as nucleoloplasts they dissolve rapidly in the medium. This problem has been solved by producing so called “giant NLBs” (Fig. 7) (12). The giant nucleoli are well visible, relatively easily handled and collected and thus ready for further analyses (12).
Figure 7. Enucleolation and production of giant NLB. (A, B): The first step of this process is the microsurgical removal of nucleoli from fully grown oocytes as nucleoloplasts. (C): Then, nucleoloplasts are aspirated into an enucleolation pipette and the piezo pulses are applied. Consequently, the nucleoloplast envelope (membrane) is broken down and NLB is freed into the manipulation medium. These steps must be then repeated very quickly with remaining nucleoloplasts. (D): Thereafter, the nucleoli are fused when contact between them is achieved and the giant NLB is produced. The image was produced by the Olympus IX71 inverted microscope. Microsurgical processes were made by Narishige micromanipulators with piezo unit (Prime Tech, Tsuchiura, Ibaraki, Japan). The sample was originated from porcine large antral follicle. Scale bar: 50.0 μm
Usually, the manipulation medium contains bovine serum albumin (BSA), which may be a problem for further, i.e. proteomic analyses. Therefore, it can be replaced with polyvinyl alcohol (PVA) (12). According to our experiences, for enucleation, the M2 medium (Zenith Biotech ZFM2-050; Czech Republic) supplemented with PVA (Sigma P8136; Czech Republic) is superior to M2 medium (Zenith Biotech ZFM2-050; Czech Republic) supplemented with BSA (Sigma A3311; Czech Republic), as the cytoplasmic membrane appears to be more compact. The enucleolation from zygotes is similar to the method reported for the oocytes (8).
CONCLUSION
It has been demonstrated very recently that zygotic NPBs are exclusively of maternal origin and are essential only shortly after the IVF or parthenogenetic activation. Thus they are probably not that essential for more advanced embryonic development. This enables much better understanding of the role(s) NLBs (NPBs) in the processes of genome activation and early embryonic development. We suppose, that deeper knowledge of the NLB/NPB composition and their role(s) in the processes of early genome activation, together with excellent encompassment of microsurgical methods of substitution of the NLBs/NPBs, may give rise to an improvement of development potential of early embryos. This will be crucial for improvement of the quality of the oocytes used in reproductive veterinary or human medicine.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.