Enterococcus faecium EM41 is an isolate from ostrich faeces. It has been genotyped by means of PCR and allotted to the species E. faecium (1, 2). This strain produces a thermo-stable proteinaceous substance, bacteriocin (Ent) EM41 which reaches the highest inhibition activity in the late logarithmic phase of growth (25 600 AU/ml). In spite of the fact that modern broiler husbandry tends to improve the efficacy of rearing with appropriate genetic crossbreeding, rearing conditions and nutrition, birds are still threatened by various diseases such as necrotic enteritis caused by Clostridium perfringens, or by salmonellosis, campylobacteriosis and/or eimeriosis, which of course leads to financial loss (3). Nowadays, animal nutritionists are looking for new ways to produce healthy food-producing animals. To prevent overgrowth of undesirable microbiota in the gastrointestinal tract of chickens, many poultry producers use probiotics (4, 5), which can beneficially influence gut microbiota and/or modify their immune system (6). Frequently, the probiotic Bacillus spp. or lactobacilli are used in broiler chickens (7). However, beneficial influences involving antimicrobial effects and immune system stimulation using probiotic enterococci and their enterocins have also been reported (8, 9, 10, 11, 12). Following up this information, our study was focused on the strain Enterococcus faecium EM41 and its enterocin (Ent EM41) to test it in a pilot/model experiment using laying hens to assess their effects on microbiota, biochemical and hematological parameters, phagocytic activity and weight gain. This strain and its enterocin have not been previously tested in animals.
MATERIAL AND METHODS
Preparing of Enterococcus faecium EM41 and its enterocin EM41 for application
The Enterococcus faecium EM41 applied was a variant treated with rifampicin prepared as previously reported by Lauková et al. (13). EM41 (0.1% inoculum) was cultivated in MRS broth (Merck, Darmstadt, Germany) for 18 h at 37 °C. The broth culture of the EM41 strain was centrifuged at 10 000 g for 30 min. The supernatant was removed and bacterial cells were re-suspended in Ringer solution (pH 7.0, Merck) to the final concentration of 109 cfu/ml; the cell count was checked by spreading appropriate dilutions of E. faecium EM41 culture onto M-Enterococcus agar plates (Difco, Maryland, USA) enriched with rifampicin (100 µl/ml, Himedia, India).
Partially-purified enterocin EM41 (Ent EM41) was prepared according to the protocol reported by Mareková et al. (14) as follows: E. faecium EM41 (0.1% inoculum) was cultivated in BHI broth (500 ml, Becton and Dickinson, Cockeysville, USA) for 18 h at 37 °C. The broth culture was centrifuged at 10 000 g for 30 min. The supernatant was precipitated with ammonium sulphate (40% saturation, for 3 hours) and stirring at 4 °C. The precipitate was re-suspended in the phosphate buffer (5 ml, pH 6. 5) and diluted in the buffer at ratio 1:1. The inhibition activity of Ent EM41 was tested according to De Vuyst et al. (15) on Brain Heart Infusion Agar (BHI Agar, Becton Dickinson) against the principal indicator strain E. avium EA5 and expressed in Arbitrary Units per millilitre (AU/ml); this is associated with the highest dilution of enterocin causing growth inhibition of the indicator strain.
Experimental birds, design of model experiment and sampling
Eighteen Lohmann Brown laying hens (aged 45 weeks) were involved in this model/pilot experiment. Regarding the birds`age, the hens had served previously as the control group in an experiment performed by our colleagues. The laying hens were fed the standard diet for 35 days as follows: 33. 5% wheat bran, 31% maize, 24.5% soy grit, 9% limestone and 2% Premix HYD 10 (Agroconsult Company, Slovakia). All care and experimental procedures involving animals were approved by the Ethical Commission of our Institute of Animal Physiology at the Slovak Academy of Science (Košice, Slovakia) and by the Slovak State Veterinary and Food Administration. The birds were placed in pens (1 animal per pen) according to the EU rules (¦ 3 NV 23/2009 Z. z.) in the approved experimental stall (SK U 07016) with access to water ad libitum. The hens were provided a light regimen of 15 h light and 9 h dark, including the dark period during the night. The temperature was kept at a value required for the age of the birds (24 – 19 ºC) and humidity was maintained in the range of 60 – 80%. The temperature and humidity were recorded continuously with a digital thermograph positioned at the same level as the pens. The pens were provided with a withdrawable part for faeces separation. The experiment lasted 35 days. The hens were divided into three groups (control group - CG; experimental group 1–EG1; experimental group 2 - EG2; 6 hens in each group, n=6). Every day at the same time in the morning, the hens in EG1 were administered the E. faecium EM41 strain (dose 400 µl/bird/day, cells concentration 109 cfu/ml) for 21 days (3 weeks). The birds in EG2 received enterocin (Ent) EM41 (40 μl/animal/day, 25 600 AU/ml). Both additives were applied to the drinking water. The diffusion of EM41 strain and Ent EM41 in the water was checked by plating of water sample dilutions onto M-Enterococcus agar (Difco, Maryland, USA) and expressed in cfu/ml. To check the microbial background, faeces were sampled (n=6) from every bird in sterile bags following the method used by Grešáková et al. (16). Sampling of faeces was carried out from each and every bird at the start of the experiment (day 0 – 1), after 3 weeks of both additives application (day 21) and at the end of the experiment (day 35, 2 weeks after EM41 and Ent EM41 cessation).
Each faecal sample was treated using the standard microbial method; one gram of faeces was mixed in Ringer solution (1:9, pH 7.0, Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) using a Stomacher Masticator (Spain). The appropriate dilutions were double plated onto selected media and cultivated at 24/48 h at 37 °C (depending on the bacteria). The following media according to the International Organization for Standardization (ISO) were used: M-Enterococcus agar (ISO 15214, Difco, USA) enriched with rifampicin (100 µg/l, to differentiate it from the other enterococci) to count E. faecium EM41 strain and without rifampicin for the total enterococcal count. Baird-Parker agar (ISO 6888, Becton Dickinson) supplemented with egg yolk tellurite solution (Merck) was used for enumeration of coagulase-positive staphylococci (CoPS); Mannitol salt agar (Difco) was used to count coagulase-negative staphylococci (CoNS). Coliform bacteria were enumerated on Mac Conkey agar (ISO 7402, Becton Dickinson, USA). Pseudomonas agar (Biomark, India) was used for Pseudomonas spp. bacteria. Campylobacter agar Karmali (Oxoid, Ltd.) was used to isolate Campylobacter spp. To check for Salmonella spp., faecal samples were inoculated into Rappaport-Vassiliadis broth (Merck, Germany) and cultivated at 37º C for 24 h. Then 100 µl of culture was plated onto Brilliant Green agar (ISO 6579, Difco). Grown colonies were picked up from BGA and genotypized by means of PCR as previously described by Herich et al. (17). To confirm the EM41 strain detected from faeces after application, the PCR method, primers and protocol according to Woodford et al. (18) were used. The bacterial counts were expressed as log cfu per g of faeces (colony- forming units per gram) ± SD.
Biochemical parameters and phagocytic activity testing
Blood sampling was performed at day 0 - 1 (start of the experiment), at day 21 (3 weeks after of E. faecium EM41 and Ent EM41 application) and at day 35 (end of experiment, 2 weeks after cessation of E. faecium EM41 and Ent EM41). The blood was sampled from the vena ulnaris into nonheparinized sterile Ependorf tubes to test the biochemical parameters: the total proteins (g/l), albumins (g/l), triglycerides (mmol/l), cholesterol (mmol/l), glucose (mmol/l), calcium (mmol/l) and phosphorus (mmol/l) using the Ransel commercial kit (Randox, United Kingdom). Blood serum was obtained by centrifugation at 3 000 x g for 10 and stored frozen in plastic vials until analysis.
Phagocytic activity (PA) was measured in heparinized blood using the direct counting procedure with microspheric hydrophilic particles (MSHP). Ingestion of MSHP by polymorphonuclear cells (PMNs) was determined with a modified test described by Vetvička et al. (19). Phagocytic activity was calculated as the relative number of white cells containing at least three engulfed particles per 100 white cells (neutrophils) and expressed as a percentage (%). Index of PA (IPA) was calculated as the number of engulfed particles per total number of neutrophils observed. The percentage of phagocytic cells was evaluated using an optical microscope, by counting PMN cells up to 100.
GPx evaluation, blood analysis and weight control
The activity of glutathione-peroxidase (GPx, EC 1.11.1.9, U/g Hb) in blood erythrocytes was determined from heparinized blood using the specific Ransel commercial test (Randox) according to the spectrophotometric assay procedure of Paglia and Valentine (20). Blood was sampled in heparinized Eppendorf tubes for evaluation of haematological parameters using a CELL – DYN 3700 hemanalyser (Abbot Laboratories, USA). Body weight was registered daily.
Statistical analysis
Statistical analyses of the results were performed using Tukey`s multiple comparison test with the level of significance set at p<0.05 ± standard deviation (SD), or Dunnett`s post test. The results were compared with control or sample collection days.
RESULTS
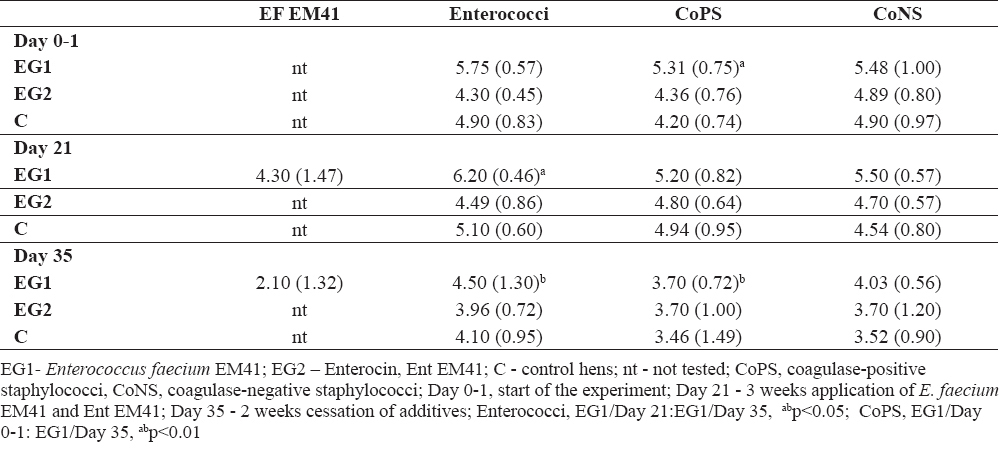
Table 1a shows counts of Enterococcus faecium EM41, total enterococci, CoPS and CoNS in the hens` faeces. Enterococcus faecium EM41 colonized the intestinal tract of the laying hens, reaching 4.30 (1.47) cfu/g at day 21 (from the initial concentration 9.0 log cfu/g).
Table 1a. Bacterial profile after Enterococcus faecium EM41 and Ent EM41 application and their cessation, expressed in colony - forming units per gram (cfu/g ± SD)
At day 35, the count of EM41 strain was 2.10 (1.32) log cfu/g. PCR genotypization confirmed the identity of the EM41 strain with the species E. faecium. Significantly higher counts of enterococci were detected in the faeces of EG1 (EM41) at day 21 compared to EG1 at day 35 (abp<0.05). The counts of enterococci were balanced at day 0 - 1. On the other hand, at day 21 higher counts (although not significant) of enterococci were found in EG1 compared to EG2- 6.20 (0.46) to 4.49 (0.86) log10 cfu/g ± SD (Table 1a). Ent EM41 could probably inhibit enterococci in EG2. Similar situation was assessed at day 35 in EG1 compared to EG2- 4.50 (1.30) to 3.96 (0.72) log10 cfu/g ± SD (Table 1a). At day 21, higher counts of enterococci were found in EG1 compared to C – 6.20 (0.46) to 5.10 (0.60) ± SD; at day 35 slight reduction of enterococci was found in EG2 compared to C- 3.96 (0.72) to 4.10 (0.95) log 10 cfu/g ± SD (Table 1a). At day 35, the counts of CoPS were significantly reduced in EG1 compared to day 0 - 1 (abp<0.01). At day 21, CoPS were slightly decreased in faeces of EG2 compared to EG1-4.80 (0.64) to 5.20 (0.82) log 10 cfu/g ± SD (Table 1a); CoPS were decreased in EG2 at day 21- 4.80 (0.64) log 10 cfu/g ± SD compared to EG1 at day 0 – 1; 5.31 (0.75) log10 cfu/g ± SD). Reduction of CoNS was also noted at day 21 in EG2 compared to EG1-4.70 (0.57) to 5.50(0.57) log 10 cfu/g ± SD); they also decreased at day 35 in EG2 compared to EG1-3.70(1.20) to 4.03 (0.56) log10 cfu/g ± SD.
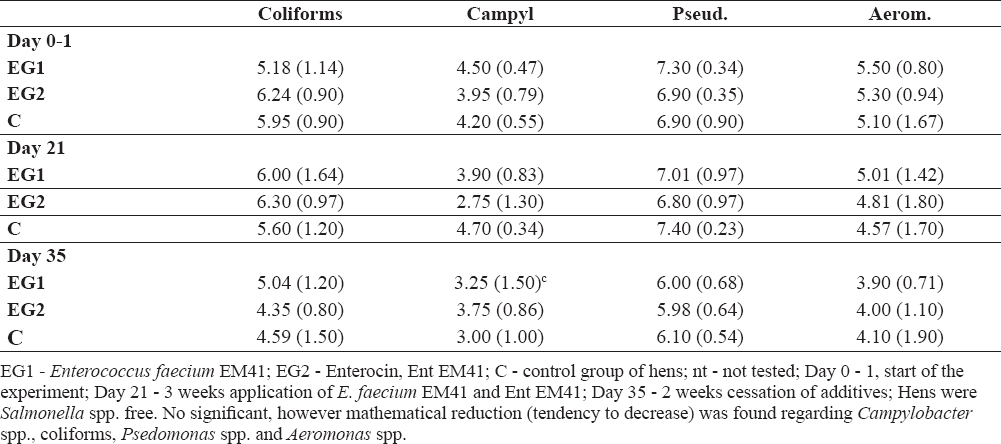
Table 1b gives the counts of coliforms, Pseudomonas spp., Aeromonas spp. and Campylobacter spp. in the hens` faeces. The hens were Salmonella spp. cells-free. Reduction of Campylobacter spp. was found in the faeces of EG1 at day 21 compared to EG1 at day 0-1; 3.90 (0.83) to 4.50 (0.47) log10 cfu/g ± SD; at day 35 lower counts of Campylobacter spp. were noted in EG1 compared to EG1 at day 0-1; 3.75 (0.86) to 4.50 (0.47) log10 cfu/g ± SD. Reduction in Campylobacter cells was also demonstrated in EG2 at day 21 compared to EG1 at day 21-2.75 (1.30) to 3.90 (0.83) log10 cfu/g ±SD; at day 21 reduction of Campylobacter spp. was indicated in EG1 compared to C-3.90 (0.83) to 4.70 (0.34) log10 cfu/g ± SD and in EG2 compared to C-2.75 (1.30) to 4.70 (0.34) log 10 cfu/g ± SD. Decrease in coliforms was noted in EG2 at day 35 compared to EG2 at day 21-4.35 (0.80) to 6.30 (0.97) log 10 cfu/g ± SD (Table 1b). Pseudomonas spp. were decreased in EG2 at day 21 compared to EG1 at day 0-1; 6.80 (0.97) to 7.30 (0.34) log 10 cfu/g ± SD; at day 35 in EG1 compared to EG1 at day 0-1; 6.00 (0.68) to 7.30 (0.34) log 10 cfu/g ± SD (Table 1b). The counts of Aeromonas spp. were lower in EG2 at 21 compared to EG2 at 0-1; 4.81 (1.80) to 5.30 (0.94); they were also decreased at day 35 in EG1 compared to EG1 at day 21- 3.90 (0.71) to 5.01 (1.42) log 10 cfu/g ± SD and at day 35 in EG1 compared to C; 3.90 (0.71) to 4.10 (1.90) log 10 cfu/g ± SD, (Table 1b).
Table 1b. Bacterial profile after Enterococcus faecium EM41 and Ent EM41 application and their cessation, expressed in colony - forming units per gram (cfu/g ± SD)
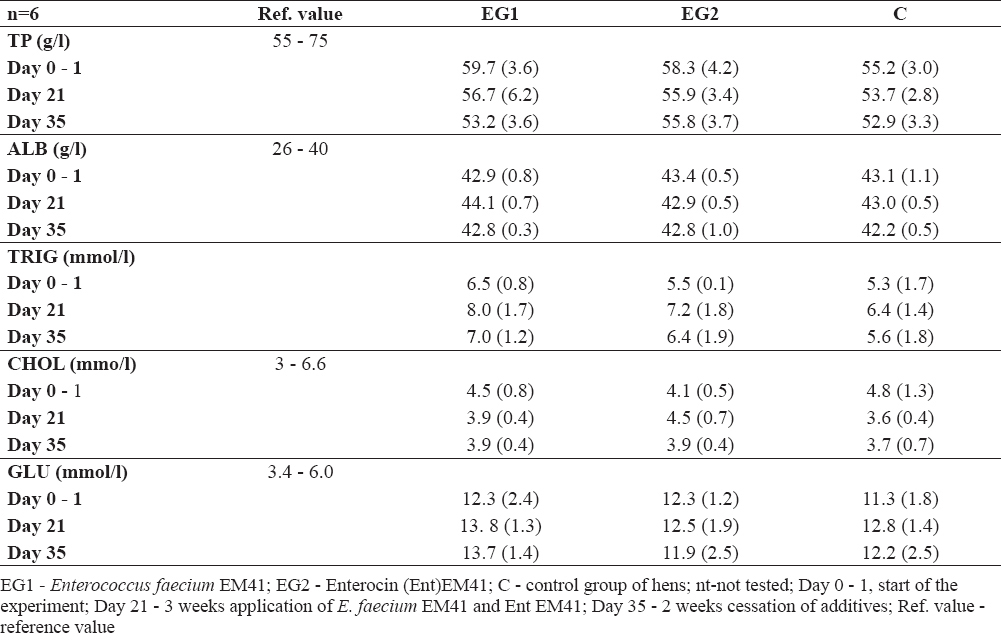
Biochemical parameters specified from the blood serum in the experimental group (proteins, albumin, triglycerides, cholesterol, glucose, calcium and phosphorus) were not negatively influenced (Table 2a, 2b). However, glucose values for instance were higher than reference values in all groups at day 35, the lowest being found in EG2 hens (Ent EM41). The total proteins, triglycerides, Ca, P were mostly in their physiological ranges. Albumin was slightly higher; however, not only in the experimental but also in the control group.
Table 2a. Biochemical parameters in sera of hens after additives application and their cessation (average ±SD)
Table 2b. Biochemical parameters in sera of hens after additives application and their cessation (average ±SD)
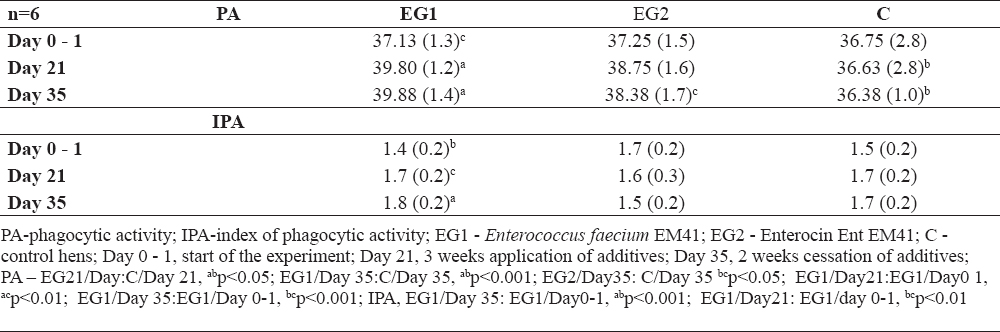
PA values were significantly increased in EG1 compared to C at day 21 (Table 3, abp<0.05). Increased PA was also noted at day 35 in EG1 compared to C (abp<0.001), as well as in EG2 compared to C (bcp<0.05). When individual samplings were compared, increased PA was noted in EG1 at day 21 compared to EG1 at day 0 - 1 (acp<0.01) and at day 35 in EG1 compared to EG1 at day 0 - 1 (bcp<0.001). The stimulation of non-specific immunity by EM41 strain was also manifested through increased IPA in EG1 at day 35 compared to EG1 at day 0 - 1 (abp<0.001) and in EG1 at day 21 compared to EG1 at 0 -1 (bcp<0.01).
Table 3. Phagocytic activity in % ± SD
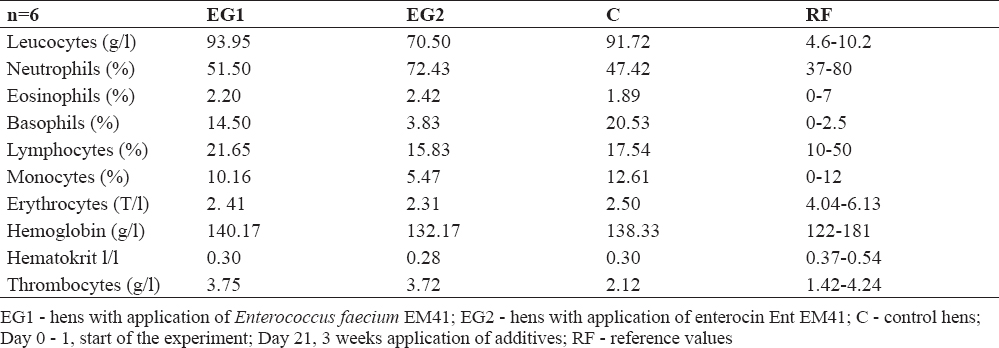
GPx values were not significantly influenced; however, lower values of GPx were measured in EG2 compared to EG1 and compared to C during the whole experiment (Table 4). The blood cell count was checked using the hemoanalyser only at day 21 (3 weeks after administration of additives). Blood cells ranged in reference values (Table 5). The weight of the hens was not influenced by additives; at day 21 only slight increase in weight was found in EG2 compared to EG1 (difference 66.7 g, Table 6).
Table 4. Values of the enzyme gluthatione peroxidase (GPx in U/g Hb ± SD)
Table 5. Blood analysis and difference in laying hens at day 21
Table 6. Weight of hens in individual sampling (in grams ±SD)
DISCUSSION
Enterococci used as probiotic strains have been found to colonize the digestive tract of various animals, involving food-producing in sufficient amounts up to 103-5 cfu/g (9, 21, 22, 23). E. faeciumEM41 followed this situation; in our case it ranged from 4.30 to 2.10 log cfu/g. Similarly, Karaffová et al. (24) reported enterocin M-producing and probiotic strain E. faecium AL41 in chickens reaching counts up to 105 cfu/g to 102 cfu/g, also in a group infected with Campylobacter jejuni CCM6191. In our study not only E. faecium EM41 itself, but also enterocin EM41 (produced by it) showed a tendency to reduce Campylobacter cells. Similarly as here with the EM41 strain, Lauková et al. (13) presented the anti-coliform effects of E. faecium AL41. The reducing effects of environmental, enterocin A (P) - producing, probiotic strain E. faecium EK13=CCM7419 has been demonstrated in the faeces and caecum of gnotobiotic Japanese quails infected with Salmonella Duesseldorf SA31 (9). Moreover, Lauková et al. (25) reported stronger therapeutic than prophylactic effects of Ent A (P) against Salmonella Duesseldorf SA31 in the digestive tract of gnotobiotic Japanese quails. In addition, Ent A (P) applied before and after the Salmonella infection had a protective effect on the duodenal epithelium. The damage to microscopic and submicroscopic structures of enterocytes and goblet cells of the intestinal epithelium and its subsequent necrosis was less intensive after treatment with enterocin A (26). The beneficial effect of poultry-derived strain Enterococcus faecium EF55 (probiotic and enterocin-producing) in broilers was also reported by Ševčíková et al. (27). Chickens infected with Salmonella Enteritidis and fed a diet supplemented with EF55 strain showed increased proliferative activity of enterocytes in the jejunum. This strain demonstrated positive impact on intestinal morphometry in the jejunum of both non-infected and Salmonella infected chickens.
Total protein, albumin and triglycerides were not influenced by the additives. Cholesterol levels have to be controlled regarding the use of hens as food animals. The main sources of cholesterol in human nutrition are animal products. This means, that the idea of reducing concentrations of cholesterol in meat and eggs is very appealing (4). Probiotic bacteria can lower cholesterol by binding cholesterol to the cellular membrane of bacterial cells, through deconjugation of bile salts which may interfere with the enterohepatic cells, or by inhibition of cholesterol synthesis (28, 29). Probiotic strain E. faecium M74 has been found to decrease cholesterol levels in humans (30). Regarding the effect of E. faecium EM41 as well as Ent EM41 on cholesterol levels in the blood serum of hens, they were not negatively influenced; they stayed in the range of reference levels. In our experiment, glucose values were higher than the reference values in all groups. However, at day 35 the lowest Glu value was found in EG2 hens (Ent EM41). It is reported that probiotic strain/substance for instance can influence the transport properties of the small intestine epithelium, and increased absorption of glucose could be interpreted as a positive effect on animals (31).
Phagocytosis is an essential component of the cellular innate immunity response and plays an important role in the host defence against infection. In this study, stimulative effect of both additives on PA was found. The beneficial effect of bacteriocin-producing and probiotic strain on PA activity was noted in our previous experiments using a rabbit model (32), but also in laying hens (13). That is, in rabbits after E. faecium AL41 application (strain producing Ent M and possessing probiotic properties), PA values reached 48.83 % ± 0.60 at the end of the experiment (day 42; in hens it was even higher - 57.0% ± 0.66). However, the highest PA was noted in broiler rabbits at the end of the experiment (day 42) - 68. 88% ± 0.9 after Ent M application; so far this is even the highest stimulation of PA influenced by enterocins (33). Karaffová et al. (24) reported modulation of TLR4, TLR21 expression and activation of MIF, IFN-β, MD-2 and CD14 in the caecum of chickens after oral administration of E. faecium AL41 to chickens and their infection with Campylobacter jejuni CCM6191. It could be stated that continuous preventive application of E. faecium AL41 during change of breeding or post hatching, for example, may stimulate the immune system of chickens. Toll-like receptors (TLRs) are pattern recognition receptors that function as microbial infection sensors and are critical for the initiation of the innate inflammatory and adaptive immune response (34). In line with this knowledge, we can supopose that the EM41 strain stimulates PA in this way as well.
GPx belongs among the most abundant antioxidant selenoenzymes protecting cells from oxidative damage (35). Assessing our results, it seems that the additives applied to our hens` diet did not evoke oxidative stress. The results of haematology indicate that additive supplementation did not influence the blood cell profile. They stayed in the range of reference values or were optimized to the reference value range. Compared to other probiotic enterococci, EM41 did not influence the hens` weight in our study (22).
CONCLUSION
It can be stated in conclusion that E. faecium EM 41 isolated from ostrich faeces and its enterocin EM41 showed antimicrobial effects through reduction of CoPS, CoNS, coliforms, Pseudomonas spp. and Aeromonas spp. PA was stimulated with significant increase after administration of the additive, but they did not negatively influence biochemical, and haematological parameters, or weight gain. Further tests using EM 41 are in process.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article