Abstract
Prenatal gross morphologic, morphometric and histologic developmental features of the dromedary spleen were studied. The dromedary gestation period (13 months) was categorized into four (1-4) phases and ten developing spleens per growth phase were sampled. Splenic topographical anatomy was noted before being eviscerated from each foetus. Morphologic and morphometric features of the eviscerated spleens were immediately documented and 2 – 4 mm thick samples were collected for histological analysis. The developing spleen was dark brown in colour, semilunar shaped and significantly increased (p<0.05) in size and weight across the four phases of prenatal development. The full-term dromedary spleen was observed tohave unique histological features. Its capsule had an inner smooth muscle and an outer predominant connective tissue layer.The pumping of stored blood from the muscular capsule and trabeculae was proportionate to the body’s requirement. The
splenic venous return was characterized by blood flow from the red pulp (venous sinusoids) to the peritrabecular sinuses, subcapsular sinuses and finally to the splenic veins. The dromedary has a sinusal type of spleen and has both open and closed types of circulation. The presence of closed circulation and absence of marginal sinus could be the reason for dromedary main health problems of blood parasites; Trypanosoma evansi. It was concluded that most of the salient features of the postnatal spleen were already evident in the first growth phase and became developed by the second phase. Other growth phases were mainly characterized by increase in sizes.
Keywords: development, dromedary, foetus, histology, morphology, spleen
INTRODUCTION
The spleen is the largest lymphoid organ (
1). It is situated in the abdominal cavity directly caudal to the diaphragm and dorsal to the rumen. Its major function is to filter blood in mammalian body systems and plays an important role in hematological and immunological functions (
1,
2). In the adult camel, the spleen is semilunar shaped (
3). It has a thick ventral line and a thin cranial line, located over the dorsal sac of the rumen, and extends to the caudal part of the kidney (
4). It has a thick capsule of 292 ± 106 mm and weighs 5.5 to 6.6 kg (
3,
4).
The mammalian spleen is surrounded by a fibrous (capsular) connective tissue from which originate the trabeculae that support the major vessels. Lymphoid tissue sheaths the minor branches of the splenic arteries to form the white pulp. Contrary to this, in rodents, the minor arterial branches terminate at the marginal sinuses. The marginal sinuses form the spaces between the white splenic pulps and the surrounding marginal zones. Whereas, others pass through the marginal zones to form the venous system of the blood-filled sinuses (red pulp) (
2).
Though many prenatal development studies have been carried out on camel organs (
5,
6), only sketchy information on the prenatal development of the camel spleen are available. There appears to be none, to the best of our knowledge, that is related to the sequential changes in the developing camel spleen. Available information on camel spleens has been on the adult spleen (
4,
7). This study was aimed at providing baseline data on prenatal gross developmental features and histogenesis of the dromedary spleen, as well as towards updating available literature in these aspects.
MATERIAL AND METHODS
Animal and study design
In an adoption of earlier categorization by Jaji et al. (
8), the dromedary gestation period (of 13 months) was categorized into four (I-IV) phases for this study. Morphologic and morphometric aspects of the work were carried out at the Maiduguri Metropolitan Abattoir, Maiduguri, Nigeria. A total of 10 developing spleens from 10 apparently normal foetuses from each phase of gestation of dromedary cows were studied. Following excision from foetal carcases, the weights (in grams, g) and dimensions (length and breadth, in centimetres, cm) of the developing spleens were measured, using a Harvard trip weighing balance (Citizen® with 0.1 g – 100 kg range) and butterfly® measuring tape (0.1-150 cm) respectively. The length was its longitudinal length including the crescent curves, while the breadth was the distance between the dorsal and ventral parts across its longitudinal axis. Means were separated by Tukey HSD test at a significance level of p<0.05.
The developing dromedary spleens were obtained after humane bleeding to death of dromedary cows. This might have an effect on the sizes of the spleens measured, as earlier observed by Zidan et al. (
4).
Sampling and processing
About 2 – 4 mm thick tissues were taken from the developing spleens at each prenatal growth phase. These samples were immediately fixed in 10% buffered formal saline, then processed using the paraffin method and sectioned to 4 µm thickness sections on histological slides, as adopted from Mahre et al. (
9). The tissues sections were stained in Haematoxylin and Eosin and later observed and captured at ×20 and ×40 magnifications of the Motic Compound Microscope BA410 (Causeway Bay, Hong Kong).
Statistical analysis
The data was analyzed using statistical software GraphPad Prism version 5.00 for Windows. The data was expressed as mean ± standard deviation. Differences between group means across the phases of development were analyzed by one way Analysis of Variance with Turkey’s Post hoc test. Differences were statistically significant when p < 0.05.
RESULTS
Morphology and morphometry of the developing dromedary spleen
The prenatal dromedary spleens were observed to be dark brown in colour and semilunar shaped (
Fig. 1). They were located ventrocaudal to the diaphragm, as an attachment on the left craniodorsal aspect of the rumen.
The first phase foetal spleen measured 7.3 ± 2.4 cm in length, 2.6 ± 0.6 cm in breadth, and 6.5 ± 4.0 g in weight (0.2% relative to the body weight). Most of these dimensions showed significant increases across the four phases of prenatal development of the dromedary spleen (
Table 1). At the last phase of gestation, the developing spleen (
Fig. 1) was found to be firmly attached to the stomach by connective tissues. The developing spleen was attached not to the diaphragm, but high in the left side of the rumen by its front lower surface. It was semilunar shaped and wider at the rear end than the front.
Figure 1. Photograph of the parietal surface of a third phase (8-10 months old) dromedary spleen. Note the semilunar shape and dark brown colour
Table 1. Mean ±SD values of spleen dimensions in the foetal dromedary
First growth phase (2-4 months)
The capsule was characterized by an outer (loose/dense irregular connective tissues with early developing stages of blood and lymphatic vessels) and inner (smooth muscle) layers (
Fig. 2). The subcapsular sinuses were evident, but the peritrabecular sinuses were not yet formed. The capsule, through its inner smooth muscle layer, was continuous with the trabeculae septae as the trabeculae and radiated the splenic parenchyma (
Fig. 2). Red pulp and white pulp areas were barely separated, but red pulp areas predominate (
Fig. 2). Lymphoid nodules had started forming in the white pulp areas,while reticular fibre layers were seen to demarcate them from the marginal zone. Penicilliary arteries (the sheathed arteries associated with blood sinusoids) were established. These were intricate and overlapped the Periarterial lymphatic sheaths (PALS) into the red pulp, then directly into the sinusoids. At the red pulp, vascular trabeculae were seen with no associated sinuses.
A B
Figure 2. Micrographs depicting the capsule (A) and parenchyma (B) of the prenatal dromedary spleen at the first phase (2 – 4 months) of development. Note that the spleen was observed to be encapsulated by an outer blend of loose and dense irregular connective tissue (ct) with developing blood and lymph vessels (dvs) and an inner smooth muscle (sm) sheaths separated from the parenchyma by a subcapsular sinus (ss). The trabeculae (T) were formed and had extended into the parenchyma. However, the peritrabecular sinuses were yet to be formed. The red pulp (rp) and white pulp (wp) areas were separable. The splenic artery (a) of the white pulp was surrounded by lymphocytes of the periarterial lymphoid sheath (PALS). Reticular fibre layers were seen to demarcate them from marginal zone (arrow). (H&E; x100 (A); x400 (B); bar=100μm)
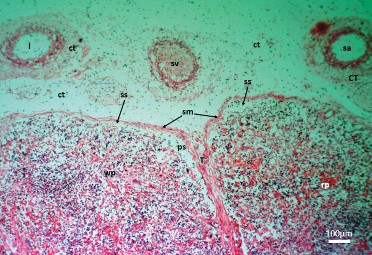
Figure 3. Micrographs depicting the capsule and parenchyma of the prenatal dromedary spleen at the second phase (5–7 months) of development. Note that the splenic capsule continued development. It was observed to be encapsulated by an outer loose/dense irregular connective tissue (ct) (vascularized with lymphatic vessels (l), small veins (sv) and arteries (sa) and an inner smooth muscle (sm) sheaths separated from the parenchyma by a subcapsular sinus (ss). The trabeculae (T) were formed and had extended into the parenchyma, the peritrabecular sinuses (ps) were well developed. The red pulp (rp) and white pulp (wp) areas were separable. (H&E; x100; bar=100μm)
Second growth phase (5-7 months)
The capsule continued development, now vascularised with blood and lymph vessels. The white pulp had lymphoid nodules aggregations and showed blood vessels (
Fig. 3). Reticular fibres were observed to demarcate them from the marginal zone, but not from the splenic red pulp. The Marginal zone could not be differentiated from the red pulp. The red pulp showed vascular trabeculae with no associated sinuses. Avascular trabeculae had started showing sinuses (
Fig. 4).
Figure 4. Photomicrographs of the prenatal dromedary spleen at the second phase (5–7 months) of development. Note that peritrabecular sinus (Arrow) had started forming around the avascular trabeculae (At). The parenchyma had red pulp (rp) and white pulp (wp) areas. (H&E; x100; bar=100μm)
Figure 5. Photomicrograph of the the spleen of a fourth phase (11–13 months) dromedary fetus depicting componrnts of its avascular trebeculae. Note that the avascular trabeculae have primary (P) and secondary (S) parts separated by peritrabecular sinuses (Arrow) (H&E; x100; bar=100μm)
Fourth growth phase (11-13 months)
All the splenic structures were developed and were typical of those of a postnatal dromedary, deferring only in sizes. This stage was associated with advancements in the development and size increases of histologic structures. The developing spleen now consisted of a thick capsule surrounding the parenchyma. Two types of trabeculae were observed: vascular trabeculae (associated with arteries, nerves, and reticular connective tissues) and avascular trabeculae (originating from the inner layer of the splenic capsule and continued to the splenic parenchyma). These avascular trabeculae were made up of two components; the core component was the primary trabeculae composed of smooth muscles and connective tissues, but with no blood vessels. The outer part was the secondary trabeculae, made up of a few smooth muscles. The two avascular trabeculae components were separated by the trabecular sinuses (
Fig. 5).
DISCUSSION
From the first prenatal growth phase, the dromedaries; spleens studied were observed to be associated to the stomach on the left side of the animals and caudal to the diaphragm, in agreement with (
10) on the positioning of ruminant spleens. However, unlike other ruminants; the dromedary spleen is semilunar in shape up to full term of prenatal life, in agreement with an earlier reported observation on the crescent-shaped spleen of adult dromedaries (
11). Chibuzo and Sivachelvan (
10) described the spleen as being a more elongated flat organ in the ox and more or less triangular in the sheep and goat. The mean dimensions and weights of developing dromedary spleen showed statistically significant increments (p<0.05 and p<0.01) across the four-phases of gestation. This is not unrelated to their prenatal hemopoietic and haematological cleansing roles (
2). The relative percentage of splenic and body weights were observed to decrease along the phases of development, due to the consistent significant increases (p<0.001) in body weight and crown- rump length, relative to the splenic weight, across the phases of prenatal development.
Histologically, the present study observed that the splenic capsule of the prenatal dromedary had outer and inner layers that were made up of connective tissue and smooth muscle respectively, right from the first growth phase and advanced in development throughout the other phases of prenatal life. A similar composition of the splenic capsule has been described in the adult dromedary (
4). The outer layer of the splenic capsule was also observed to be made up of collagen, elastic and reticular connective tissue fibres with less smooth muscle cells interwoven with the outer connective tissues (
4). Similarly, the equine splenic capsule was made up of an outer and an inner thin layer of thick connective tissue and smooth muscle fibres, respectively. On the contrary, however, ruminants have been observed to have two layers of thin smooth muscle fibres. In the pig the capsule is formed mainly from smooth muscle, whereas in the dog and cat, the smooth muscle makes up about two third of the capsule thickness (
12). The human splenic capsule is made up of connective tissue with very few smooth muscle fibres (
2).
Comparatively, the splenic trabeculae of the dromedary were observed to be extended from the capsular region up to the parenchyma (like other species), but differs in that it is divided into vascular and avascular trabeculae. The vascular trabeculae had arteries and nerves devoid of veins. The vascular trabeculae appeared to be lesser in number than the numerous avascular type. The avascular trabeculae were further divided into primary and secondary trabeculae. Typical of pig, the secondary trabeculae is made up of smooth muscle and interposed with the red pulp (
13). The red pulp and white pulp areas were separable as early as the first phase of prenatal histogenesis of the spleen, but red pulp areas predominated. This dominance was maintained in the other phases. Erythropoiesis is a major function of the developing spleen, and splenic erythropoiesis persists in new-born horses and ruminants for several weeks postpartum (
12).
This study observed that trabeculae veins and splenic capsular were respectively replaced by peritrabecular and subcapsular sinuses right from the first and second growth phases and they became more conspicuous with advancing prenatal growth phases. Peritrabecular sinuses are features in adult dromedary spleens (
4,
14). Collecting veins similar to large sinuses were reported to extend under the capsule (
15). These structures are unique with the dromedary.
At the last prenatal growth phase, blood sinusoids were observed to divide the red pulp parenchyma into cords. The parenchyma got splitted by the secondary avascular trabeculae with an irregularly distributed form within the red pulp. These concurred with an earlier description of the arrangement of red pulps of the adult dromedary spleen (
14,
15). The splenic parenchyma is made up of two major parts, red pulp and white pulp. The first major part (red pulp) continued with the splenic cords and split to small compartments by the secondary trabeculae, while the white pulp was made up of periarterial lymphatic sheaths (PALS) and a spherical lymphoid nodule, sometimes indented at the site of the PALS (
4). The secondary follicles had a corona surrounded germinal centres. The central portion branched into a maximum of 4 straight branches of (Penicilliary) arteries) and further branches, following, its emergence from the PALS (
4). The extensive redistribution of the secondary avascular trabeculae within the sinusoids and splenic cords exacerbated the quick outflow of blood when spleen got contracted (
4). This anatomical pattern of the prenatal establishment of the muscular capsule and trabeculae of the dromedary spleen portends its contractile function. The dromedary spleen stands as a contractile store that forces its stored blood out for circulation. The pumping of stored blood from the muscular capsule and trabeculae is proportionate to the body, as is required, such as in extreme physical activities and haemorrhagic conditions. Based on the blood content of the sinusoid within the splenic red pulp, the dromedary spleen is defined as sinusal type (
4). This contrasts from the non-sinusal type found in other ruminants (
12). The dromedary spleen stores blood, in a similar manner to the other sinusoidal types of spleens of the dog, horse and pig (
12).
At the last prenatal growth phase, the white pulp of the dromedary spleen was delineated by reticular fibres (circumferential) that resulted in its division into two (
2) parts, one surrounding the lymphoid follicles and the other in form of a network at the periarterial lymphoid sheaths (PALS). A similar finding has been reported in mice and man (
16,
17).
Reticular frame work plays a vital role in lymphocytes arrangement and compartmentalization, which is typical for the camel white pulp. Comparatively, the dromedary white pulp is completely surrounded by the marginal zone, similar to most species (
12), with the exception of human beings where the marginal zone surrounds the lymphoid follicles only (
18). Seki and Abe (
19) noted that the arterial capillary extended to the end of the periarterial macrophage sheaths (PAMS) in the red pulp, seen commonly in the cattle, dog, horse, pig, and rat, with the exception of a cat. The arterial capillary drained directly to the sinusoids or splenic cords. Thus, giving the dromedary spleen a dual, open and closed circulations as found in dog and cat (
19) and in man (
20). Conclusively, most of the salient postnatal features of the dromedary spleen were already evident in the first growth phase and were established during the second growth phase. Other growth phases were characterized mainly by increase in sizes.
The dromedary sinusal spleen functions as to store blood right from the prenatal life. The pumping of stored blood is the function of the thick muscular capsule and trabeculae which is proportionate to the body’s requirements (
4). The dromedary spleen is found to have dual, open and closed circulations. The venous return pattern of the dromedary spleen is also unique, in that the blood flows from the red pulp (venous sinusoids) to the peritrabecular sinuses, subcapsular sinuses and splenic vein (
4). It was speculated that the absence of the marginal sinus and the presence of a closed circulation in the dromedary spleen is due to reduction of the marginal zone in blood filtration, which could be a reason why the blood parasites (e.g. Trypanosoma evansi) become the major health problem in dromedary camel.
CONCLUSION
Conclusively, most of the salient gross and histologic postnatal features of the dromedary spleen are already evident in the first prenatal growth phase and are established during the second growth phase. Other growth phases are characterized mainly by increase in sizes.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conf lict of interest with respect to the authorship and/or publication of this article
ACKNOWLEDGEMENT
This was a collaborative study between the Faculties of Veterinary Medicine of two Nigerian universities, the University Maiduguri and the University of Ilorin. We duly appreciate the support of the managements and the technical staff of these faculties and the management of the Maiduguri Metropolitan Abattoir, Maiduguri, Nigeria, for their kind support.

 10.2478/macvetrev-2019-0018
10.2478/macvetrev-2019-0018