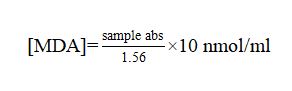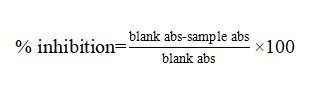Abstract
The present study was aimed to evaluate hematological and oxidative stress parameters in domestic dogs infested naturally (n=10) by Rhipicephalus sp. to compare with non-infested dogs (n=10). All blood samples were collected from brachial vein into tubes EDTA for the hematological analysis such as red blood cells (RBCs), white blood cells (WBCs), hemoglobin (HGB) and platelets (PLT). Serum was rapidly separated after centrifugation and stored at -20 °C until it was used for malondialdehyde (MDA) and 2,2’-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) inhibition measurements. HGB in non-infested dogs was significantly higher than in infested dogs (P<0.05). There was not a significant difference in RBCs, WBCs and PLT between bothgroups (P>0.05). The mean of MDA concentration was high in infested dogs. (0.92±0.62 nmol/ml) compared to non-infested dogs (0.75±0.25 nmol/ml). On the other hand, the percentage of ABTS inhibition was similar in both groups (P=0.71). High tick number seems significantly affected WBCs (P<0.0001) and HGB (P<0.001) in infested dogs. Concerning oxidative status, there is no significant differences (P>0.05) between low and high infested dogs neither in the amount of MDA nor in the ABTS inhibition. In conclusion, infested dogs induce RBCs alterations, which coincides with the oxidative damage, as evidenced by MDA serum levels. Also, there was a relationship between the tick number in infested dogs and the hematological parameters.
Keywords: Rhipicephalus sp., hematological parameters, oxidative status, dogs
INTRODUCTION
Ticks (Ixodidae) are arthropods that live as blood-sucking ectoparasites (
1). Hard ticks cause major health problems in dogs and transmit an important number of diseases to other animals and to humans as well, such as
Babesia,
Theileria and
Anaplasma spp. (
2). They attach to the dog's body and their bites cause irritation, redness, itching, auto-traumatic reactions and anaemia (
3). A recent study in Algeria (Bejaia province) demonstrates that
Rhipicephalus sp. are the most prevalent species in domestic dogs (
4). The most damaging impact of tick bites is the release of neurotoxins from tick saliva causing dog paralysis, systemic diseases and hypersensitivity reactions (
5). These neurotoxins can interfere with acetylcholine at the neuromuscular junction, resulting in the release of neuromuscular blockage (
6). Oxidative stress corresponds to an imbalance between the rate of oxidants production and that of their degradation in favor of the excessive generation of free radicals and other reactive oxygen species (ROS) (
7). Several scientific papers reported that the primary or secondary cause of animal diseases are due to an oxidative damage of tissues and cellular components (
8). These play an important part in tissue involvement in a number of diseases processes (
9). Free radicals are high-reactive substances that are continually produced during metabolic processes. Excess free radicals cause alterations in DNA, enzymes, and membranes, and lead to changes in immune system activity (
10). Lipid peroxidation is a mechanism of cell damage used as an indicator of oxidative stress in body fluids of cells and tissues. Lipid peroxides are unstable compounds that give a complex series of reactive carbonyl compounds when they decompose. Polyunsaturated fatty acid peroxides produce a monodialdehyde (MDA) during decomposition (
11). Moreover, the mensuration of ABTS (2, 2-Azinobis (3-ethylbenzothiazoline)-6-sulphonic acid) levels is one parameter to evaluate oxidative stress status.
Several studies confirmed the occurrence of oxidative stress in animals infected with parasites (
12,
13). There would be important changes in the hematology and biochemistry of hosts suffering from parasitic infestation depending on the parasite species and the hosts sites (
14). These hematological and biochemical of blood can help in diagnosing the severity of parasite infestation and evaluation of management practice and physiological status of animal (
15). To our knowledge, there (are few of) is not a sufficient literature on hematological and oxidative stress values on infested animals by parasites especially those infested by ticks. We hypothesized that
Rhipicephalus sp. would alter specific hematological and oxidative stress indices in infested dogs. Therefore, this present study was aimed to evaluate of hematological and oxidative stress parameters in domestic dogs infested naturally by
Rhipicephalus sp. ticks (Ixodidae).
MATERIAL AND METHODS
Animals and samples
The study protocol was approved by the Scientific Committee of the Faculty (University of Bejaia). Blood sampling of the dogs was carried out following the rules of well established veterinary practice. The present study was conducted on dogs presented in veterinary clinic situated in
Bejaia province (36°43’N, 5°04’E) for different reasons (care, vaccinations, etc.) from April to July 2016. This study was carried out on two animal groups of various breeds (n=20 with mixed sex and ages). During the study period, infested dogs with
Rhipicephalus sp. and healthy dogs were selected randomly from different habitats (home and farmhouse) after a dermatological examination. The number of collected ticks was recorded for each dog. All ticks were removed carefully manually by using forceps in order to keep the rostral implantation intact and were stored in labelled plastic containers containing 70° ethanol. Each tick was identified using a stereomicroscope (MOTIC, ST-37C-2LOO) according to the standard morphological identification keys (
16).
All blood samples were collected from the brachial vein into tubes containing EDTA as an anticoagulated for the hematological analysis. Serum was rapidly separated after centrifugation at 1200 rpm for 20 min and stored at -20 °C until use for oxidative status measurements.
Hematological and oxidative status measurement
Hematological indices measurement such as red blood cells (RBCs), white blood cells (WBCs), hemoglobin (HGB) and platelets (PLT) was carried out using automatic blood counter (SWELAB alfa, Boule Medical AB, Spanga, Sweden).
Thiobarbituric acid reaction substances (TBARs) assay
Serum MDA concentration (nmol/mL) was measured according to the method of Sivonova et al. (
17), with some modifications. In order to precipitate protein, 1ml of the serum sample was added to 0.5 ml of trichloroacetic acid (TCA 30%) and incubated at 0 °C during 2 h. The mixture was centrifuged (3000 x g for 10 min at 4 °C). After then, 1 ml of the supernatant was mixed in 0.25 ml of thiobarbituric acid (TBA, 1% diluted in 0.05 mol/l NaOH) and 0.075 ml of EDTA (0.1 mol/l) in a glass tube and placed into a boiling water bath (95 ºC) for 15 min, and immediately cooled in an ice bath (0 ºC) to stop the chemical reaction. The thiobarbituric acid reactive substances (TBARS) were then quantified using a spectrophotometer (Biotech Engineering Management Co. Ltd. UK VIS-7220G) at a wavelength of 532 nm. The estimated MDA rate (nmol/ml) was calculated using the formula:
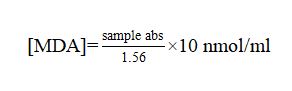
Total antioxidant capacity (TAC) assay
The total antioxidant capacity was measured with the radical cation decolorization assay (18). This assay is based on the inhibition by antioxidants of the radical cation absorbance of diammonium salt ABTS. In brief, ABTS was dissolved in deionized water to produce a solution of 7 mmol/l concentration. ABTS+ was generated by mixing the ABTS stock solution with 2.45 mol/l of potassium persulfate, and the mixture was left in the dark at room temperature for 12-16 h. The ABTS+ solution was dissolved with PBS at pH 7.4 to an absorbance of 0.7 (±0.02) at 734 nm. After adding 2 ml of ABTS+ diluted to 20 μL of sample in PBS, the absorbance was taken exactly 6 min after the initial mixing. PBS was used in each test as blank. The free radical trapping capacity of the biological sample was expressed as the percentage inhibition of ABTS+. The data obtained were used to determine the ABTS+ inhibition (%) using
the formula:
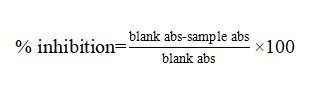
Statistical analysis
Data were analysed using a mixed model for repeated measurements using Statview Software, Version 4.02 (Abacus concepts Inc., Berkeley, CA, USA). Statistical analysis was performed using Fisher’s test to compare infested and non-infested dogs. The values significance was expressed as mean ± SD, and P<0.05 was considered significant.
RESULTS
In this study, a total 55 matured hard ticks (41 males and 14 females) were collected and identified from domestic dogs. The microscopic observation revealed of
Rhipicephalus sanguineus,
Rhipicephalus bursa and
Rhipicephalus turanicus based on external morphological characteristics (
Fig. 1).
Figure 1. Ticks infestation on dogs. Rhipicephalus turanicus male (A1, A2), Rhipicephalus turanicus female (A3, A4), Rhipicephalus bursa male (B1, B2), Rhipicephalus bursa female (B3, B4), Rhipicephalus sanguineus male (C1, C2) and Rhipicephalus sanguineus female (C3, C4) with dorsal and ventral face, respectively
The mean ± SE values of hematological parameters of infested dogs by
Rhipicephalus sp. and non-infested shown in
Table 1. HGB in non-infested dogs were significantly higher than in infested dogs by
Rhipicephalus sp. (P<0.05). There was not a statistically significant difference in RBCs, WBCs and PLT between both groups (P>0.05). The mean values of RBC and PLT were practically similar in infested and non-infested dogs.
Fig. 2A and
2B illustrate the MDA concentrations and ABTS+ inhibition in infested by
Rhipicephalus sp. and non-infested dogs, respectively. The mean of MDA concentration was high in infested dogs by
Rhipicephalus sp. (0.92±0.62 nmol/ml) compared to non-infested dogs (0.75±0.25 nmol/ml) (P<0.01). On the other hand, the percentage of ABTS+ inhibition was similar in both groups (P=0.71).
Figure 2. MDA concentrations (A) and total antioxidant capacity (B) in non-infested and naturally infested dogs by Rhipicephalus sp.* Significant difference in value levels between non-infested and infested dogs (P<0.05)
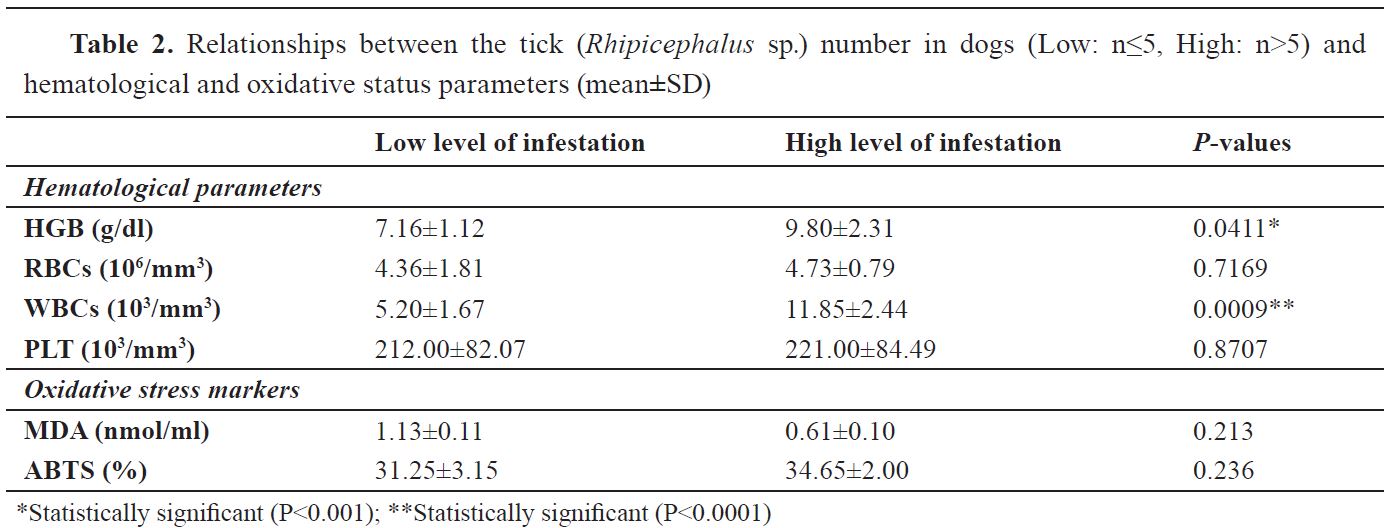
The results of relationships between the tick number in dogs (Low: n≤5, High: n>5) and hematological and oxidative status parameters are presented in
Table 2. High tick number seems significantly affected WBCs (P<0.0001) and HGB (P<0.001) in infested dogs. With regard to RBCs and PLT, it was not affected by the tick number in infested dogs. Regarding oxidative status, there are no statically significant differences (P>0.05) between low and high infested dogs neither in the amount of MDA nor in the ABTS+ inhibition.
DISCUSSION
Many physiological situations may alter the physiological equilibrium that is maintained mainly by the blood in the body (
19). Medical analysis is an important tool that helps veterinary practitioners to successfully diagnosis the disease in a fast way and to a better understanding of their impact on animal health. Modifications in biochemical and hematological values of blood can help in the understanding of the physiological and pathological status of animal (
20). Antioxidants are chemicals that inhibit the oxidations of other chemicals. They are involved in preventing cellular damages from oxidative stress and also lower the risk of chronic diseases (
21). It is well-documented that the balance between oxidants and antioxidants may be impaired the physiology of animals. The present study was conducted in order to assess the hematological and oxidative stress indices in domestic dogs infested naturally by
Rhipicephalus sp. ticks compared to non-infested dogs.
Anemia is functionally defined as decreased oxygen carrying capacity of blood, this condition is clinically characterized by reduction in the hemoglobin, hematocrit or total erythrocyte count (i.e. RBC) per unit volume of blood in a normally hydrated animal (
22). In the present study, the comparison of some hematological parameters between both groups revealed that leucocyte count (i.e. WBC) and hemoglobin were affected negatively in infested dogs which could be expressed by a clinical sign, namely anemia due to blood sucking by the ticks. It is also due to destruction of RBC’s by the protozoan parasites (
Theileria, Babesia) transmitted by ticks. It has been reported
Theileria infection in cattle resulted in anemia associated to significant decreases in total erythrocyte count, hemoglobin concentration packed cell volume and platelets count (
23). A similar observation was reported by other researchers in their study, babesiosis and theileriosis induced significant decreases in RBC, HB and PCV compared to the control ones which, indicated severe anemia (
23,
24). The results obtained by (
25) corroborate our results which showed a significant wide difference in leukocytes levels in dogs with babesiosis (10.5 x 10
3/mm
3) and control dogs (4.6 x 10
3/mm
3).
In the other investigation, (
26) it was indicated that the platelet count of cattle parasitized by ticks was significantly higher (P<0.01) as compared to that in the non-parasitized cattle.
Recently, Kaur et al. (
26) showed that there was a significant reduction in the hematological parameters of cattle following heavy tick burdens. Our finding is in agreement with those reported by Pfäffle et al. (
27) which thrombocyte counts, i.e. PLT, in the infested population they were directly correlated with the tick burden. In addition, Tinoco-Gracia et al. (
28) considers that the parasite intensity is severe when the number of ticks on the host exceeds 30. The results of the present study revealed that the dogs were anemic and dehydrated as compared to non-infested dogs due to heavy infestation of ticks. It is important to remember that anemia and inflammatory stimuli influence the release of thrombocytes from the spleen pool or the bone marrow. Moreover, the decreased lymphocytes can be an indication of an inflammatory or an immune response due to pathogen infection (
29).
The results of the current research are consistent with preceding scientific report where changes in the antioxidant system have been demonstrated with a variety of parasite infestations in animals (
30). This is supported by Singh et al. (
31) who demonstrated that significant alteration in oxidant/antioxidant balance may be implicated in pathogenesis of clinical Sarcoptic mite infestation. The results obtained by Ellah et al. (
23) and Al-Hosary et al. (
13) on the influence of parasitism by
Theileria sp. on MDA levels (P<0.0001) in cattle and sheep have shown that parasitism causes oxidative stress in the hosts. Similar observations have been reported by Crnogaj et al. (
32) which observed a decrease in antioxidant biomarkers (SOD, GPx and catalase) in dogs naturally infected with
B. canis canis. Kiral et al. (
33) reported that MDA level in dogs infested by
H. canis was high than in controls. Also, another investigation showed that there was a significant increase in serum MDA levels (P<0.0005) between the healthy control group (8.13±1.78 μmol/l) and dogs with diagnosed babesiosis (36.90±13.95 μmol/l) (
34). In the present study, the height in serum lipid peroxidation product (MDA) indicated the occurrence of oxidative stress in infected dogs that may be considered as an indication of cell injury caused by
Rhipicephalus sp. This may be related to an incapacity of the antioxidant mechanism to evacuate reactive oxygen species. It is known that total antioxidant status evaluates the antioxidant activity of the organism in a global way (
35). So, it is noted that the presence of parasites affects the systems of antitoxic defenses by increasing or decreasing their concentrations, but also by inhibiting or activating their synthesis (
36). In the current investigation, the level increased of TAC in dogs infected by
Rhipicephalus sp. may probably be ascribed to the production of antioxidant enzymes as free radical scavengers during the oxidative process. It has also been demonstrated by that the presence of parasites could modify host defense systems; the same authors recorded an increased activity of antioxidant enzymes in
Cyprinus carpio infected with cestode
Ptychobothrium sp. (
37). Recently, Al-Hosary et al. (
13) reported a significant increase in serum TAC in ewes infected with ticks. These results agree with those reported earlier in sheep suffering from babesiosis (
38), and in canine babesiosis compared to controls (
32). Furthermore , Ciftci et al. (
39) observed that TAC levels decreased in
Babesia vogeli infected dogs but these variations were not found as statistically important (P>0.05). However, Crnogaj et al. (
34) obtained a TAC values significant low in diseased dogs with
Babesia canis canis compared to controls. This difference could be justified by the limited number of samples collected in the current investigation and can also be attributed to other physiological factors in animals such as gestation and lactation, and age, which is coupled with the releasing of free radicals in the body and constitutes a further source of oxidative stress (
13).
CONCLUSION
Based on the results of the present study, it was concluded that infestation by Rhipicephalus sp. in infested dogs induces hematological indices alterations which coincides with the oxidative damage, as evidenced by MDA serum levels. Also, there was a relationship between the tick number in infested dogs and the hematological parameters analyzed. Further studies geared toward should be strengthened by on larger number of dogs and to determine the risk factors in order to develop effective disease control strategies.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no potential conflict of interest.
ACKNOWLEDGEMENTS
AUTHORS’ CONTRIBUTIONS
RK carried out the experimental work and wrote the manuscript. OB and MBK reviewed the manuscript. LH contributed in the sampling. AA designed, supervised the experimental study and reviewed the manuscript. All authors read and approved the final manuscript.

 10.2478/macvetrev-2020-0022
10.2478/macvetrev-2020-0022