Tick-borne diseases are highly prevalent in domestic and wild ruminants and they may be distributed in wide geographical ranges by animal transportation. The aim of the current study was to investigate the presence of European strains of Babesia spp. and/or Anaplasma spp. in oversea imported reindeer specimens. Imported specimens (n=7) were hospitalized with visible tick infestation (Ixodes ricinus) and signs of cachexia, anemia, and hemoglobinuria. Using blood smears, PCR, and BLAST comparisons, it was confirmed that the animals were infected with a French strain of Anaplasma phagocytophilum and Babesia divergens which is considered to be absent in the USA. We conclude that oversea importation of reindeers must be followed with a routine check for geographically-specific strains of pathogens from the place of origin. This monitoring process must be dynamic and according to recent reports of tick-borne pathogens.
Babesia divergens,
Babesia spp. EU1,
Borrelia (
B. burgdorferi s.l.),
Anaplasma (
A. phagocytophilum) and
Mycoplasma wenyonii (or other haemoplasma closely related to) are the main pathogens transmitted by
Ixodes ricinus to ruminants in Europe (
1,
2,
3,
4). These
Ixodes-related pathogens are geographically related to forest pastures are therefore most highly present in wild-animal ruminant species (
5,
6). Transport of such animals may contribute to wide dispersion of local geographical strains of these pathogens. Babesiosis in reindeer has been related either to
Babesia odocoilei (USA) or
B. divergens (Europe) (
7), or more recently to
Babesia spp. EU1 (also known as
Babesia venatorum) in the Netherlands (
8). Moreover, up to 5 different
Babesia species (
B. venatorum,
B. capreoli,
B. capreoli-like,
B. odocoilei-like and
B. divergens) have been identified in asymptomatic captive reindeer in Germany (
9). Infection by
Anaplasma spp. is less reported despite the fact that
A. phagocytophilum and
A. ovis can infect reindeer (
10,
11,
12).
We hypothesized that French strains of
Babesia spp. or
Anaplasma spp. may be diagnosed in imported, tick infested or non-infested reindeer specimens from an oversea geographical origin. Therefore, the aim of the current study was to investigate the correlation between severe clinical manifestation in imported reindeer specimens from the USA infested by
Ixodes spp. and concomitant infection with
Babesia spp. or
Anaplasma spp.
MATERIAL AND METHODS
The study was conveyed on seven adult captive reindeers (
Rangifer tarandus tarandus) that were hospitalized with medical history of anorexia, depression, pyrexia, and significant loss of weight. 4 weeks prior the hospitalization. Three were admitted dead, two died within a few hours after admission, and two were successfully treated. The deceased animals were processed on necropsy examination.
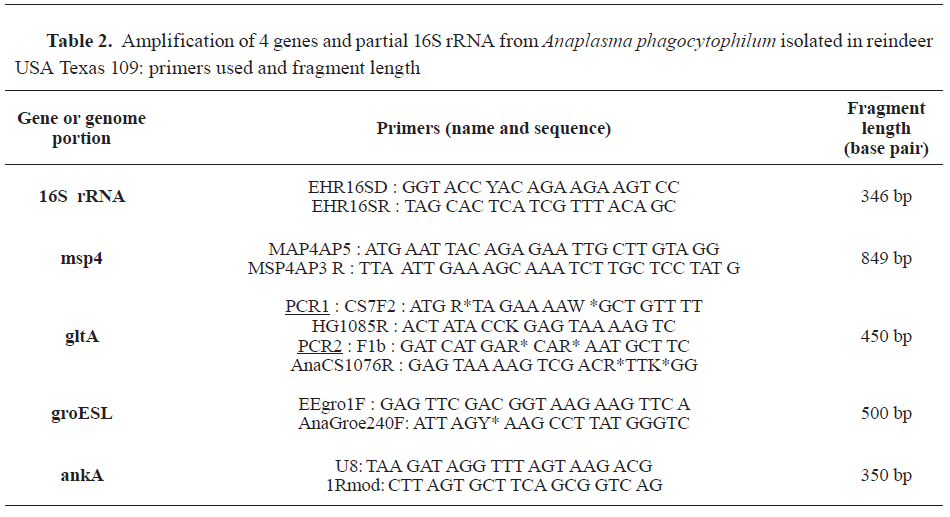
Blood smears (Microscope Nikon type 104, 40x) and coprology were performed on the treated animals. Blood cell count, hematocrit and hemoglobin were also performed (Hematology analyser XT 200 iV, Sysmex France, F-95944 Roissy). Multiple PCRs on blood samples was performed for
Anaplasma (
1,
12,
14,
15) and
Babesia (
16), followed by sequence analysis and BLAST comparison with sequence databanks.
DNAs were extracted from 200 μl of EDTA blood samples collected from reindeer using a QIAamp DNA Blood Mini Kit (QIAGEN® France-91974 Courtabeuf), eluted with 100 μl of buffer AE and stored at – 20 °C until use.
For
Anaplasma spp., 5 distinct PCR assays were employed through two steps (as described in
12,
14, and
15). First, the DNA extracts were screened with broad-spectrum PCR primers targeting 16S rRNA gene of
Anaplasmataceae, and then with primers targeting major surface protein 4 gene:
msp4. Secondly, positive samples were subjected to confirmation by 3 amplification assays: PCRs targeting
groESL heat shock operon,
ankA gene, and citrate synthase gene:
gltA. For
Babesia a genus-specific PCR based on the amplification of a fragment of an 18S rRNA gene was performed, using BJ1 and BN2 primers (as described in
16).
PCR assays were performed on an Eppendorf® Mastercycler ep-Gradient thermocycler (Eppendorf France-78360 Montesson).
PCR products were analyzed by gel electrophoresis in 2% agarose (SYBR® Safe DNA gel stain, Invitrogen, Carlsbad, USA).
Sequence analysis was performed with NCBI blast tools (see:
https://blast.ncbi.nlm.nih.gov/).
RESULTS
Clinical examinations revealed cachexia, dehydration (estimated >10%), polypnea (>50 movements per minute), tachycardia (>90 beatings per minute), and hemoglobinuria. The color of mucosae was normal to pale, without signs of icterus. Tens to thousands of engorged
Ixodes ricinus were found on each animal (from reindeer 172: 34 ticks, to reindeer 282: countless >1000 ticks).
Blood analysis revealed moderate anemia (
Table 1).
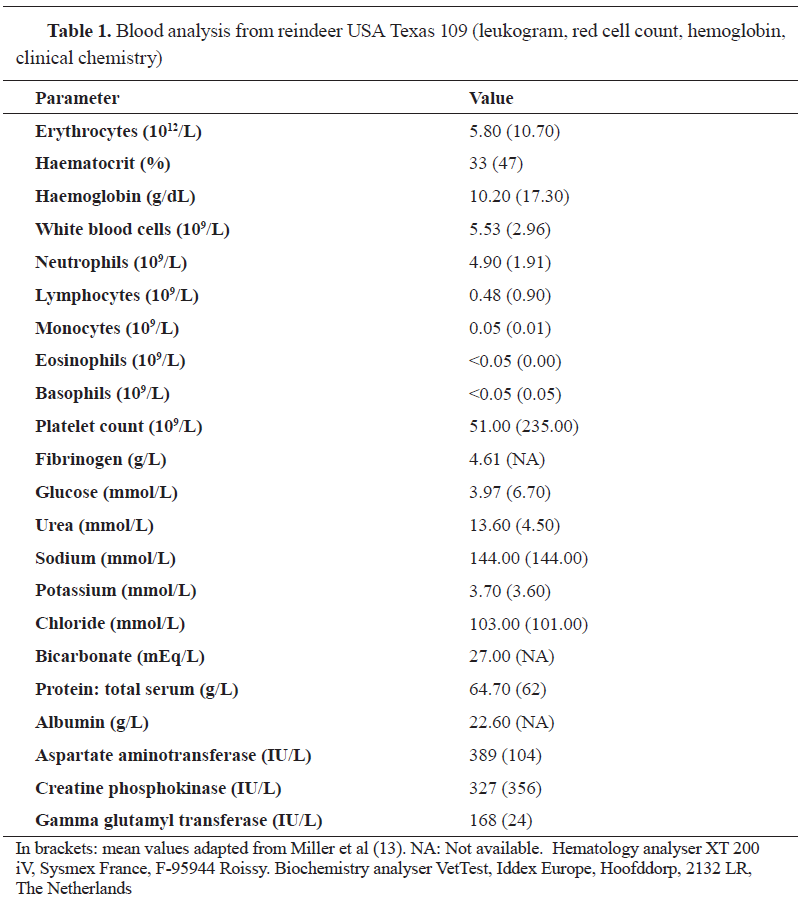
Blood smears revealed intra-erythrocytic protozoa morphologically identified as Babesia divergens (
Fig. 1).
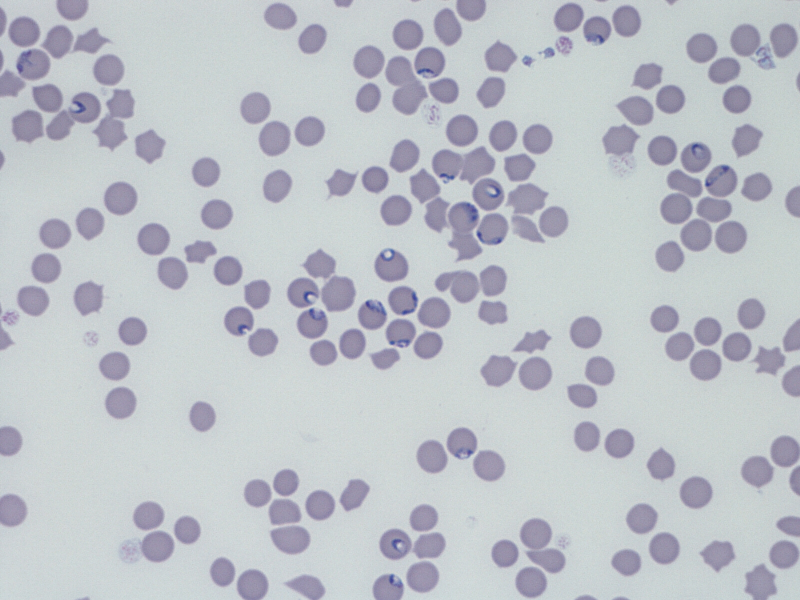 Figure 1.
Figure 1. Multiple protozoa (arrows: pear-shaped intra-erythrocytic parasites) in a blood smear (from reindeer USA Texas 109) identified as
Babesia divergens. Microscope Nikon type 104, 40x
Another blood smear a few hours after revealed the presence of intragranulocytic morulae identified as
Anaplasma phagocytophilum (
Fig. 2).
 Figure 2. Anaplasma phagocytophilum
Figure 2. Anaplasma phagocytophilum morula (arrow: blackberry-shaped intracytoplasmic foreign body) in a neutrophilic granulocyte (reindeer USA Texas 109). Microscope Nikon type 104, 100x
PCR results confirmed the morphological, bacteriological (
Table 2) and parasitological findings (data not shown). After sequencing 5 genes of
A. phagocytophilum (Genbank accession numbers JX841250 to JX841254, respectively), BLAST analyses of the sequences confirmed that the isolate was 100% identical to a French
A. phagocytophilum isolate (strain BOV 10_179 CCXQ01000001 as deposited in the European Nucleotide Archive (see:
https://www.ebi.ac.uk/ena/data/view/CCXQ01).
DISCUSSION
We found that all animals suffered from coinfection with the two major pathogens transmitted in Western Europe to ruminants by
Ixodes ricinus (
17). Poor nutritional condition, parasitism and transport-related stress (such as “shipping fever” documented in cattle) were the factors contributing to the severity of the disease.
Babesia spp. infection in reindeer is not considered to have frequent clinical manifestation. In the USA,
B. odocoieli is correlated with а high mortality rate in reindeers (
18), but it can affect other wild ruminants as well (elk - Cervus elaphus elaphus, and white-tailed deer - Odocoielus virginianus) (
19,
20). A larger Babesia has also been described in one fatal case in a reindeer (
20).
B. divergens infects ruminants in Europe but has never been reported in the USA.
B. divergens-like/MO-1 in the USA is a distinct strain of
B. divergens, which has a lower infection rate in cattle, and distinct morphology when grown
in vitro (
21,
22,
23).
In early reports in Europe, infection of roe deer with
Babesia spp. was supposed to result in subclinical to mild symptoms (
11,
24). It is possibly due to the lack of exposure to tick bites under natural conditions, as reindeers seem to be extremely sensitive to most tick-borne pathogens (
7,
19). In Europe, babesiosis in reindeer is induced by the very common
B. divergens (
7), but some protozoa close to
B. odocoieli have also been detected in European ticks collected on wild cervids (
5,
24). A larger
Babesia (
B. jakimovi) has also been described in naturally infected reindeers in Siberia. The zoonotic large
Babesia spp. EU1 has been reported in
Ixodes ricinus in a roe deer (
3,
25,
26,
27), and in a reindeer (
8).
B. divergens,
Babesia sp. EU1 and
B. odocoieli are genetically and/or morphologically similar (
3,
26,
27,
28). Many of “
Babesia divergens” infections in European cervids may have been also caused by
B. capreoli (
3,
29). Hence, formal molecular identification of
Babesia sp. in wild ruminants is routinely performed (
12).
Anaplasma ovis infection may result in a severe disease in reindeer (
10). The vectors associated with this bacterium are not
Ixodes spp (
30). In our case, no specific sequence of the bacterium was found (
30).
Disease due to
Anaplasma phagocytophilum infection has not been fully described in reindeer, even if the infection seems moderately prevalent (
12). The infection in domestic and wild ruminants results in a seldom fatal disease unless complicated by other infections (
2,
31). In our clinical observations, we hypothesized that the severe clinical manifestations in reindeer are a result of coinfection with
Babesia divergens. Contributing factors were the supposed immune naivety and theshipping stressors.
Anaplasma phagocytophilum and/or
Babesia divergens are frequently related to
Ixodes ricinus infestations in Europe. The findings for
Anaplasma range from 2 to 45% in various European countries (
12,
32), for
Babesia divergens from 0.9 to 6.7% (
33), for both pathogens from to 4 to 8% in Germany (
12,
34), and up to 46.7% in Poland (
6). These rates are correlated with the tick life-stage and sex, detection method, and geography (country) (4). The tick population in one geographical zone may contain one or more infective agents (
6,
17,
35). The transmission cycle of
Anaplasma phagocytophilum is not fully elucidated in Europe. If the documented vector is
Ixodes ricinus, the reservoir is not precisely known (
31,
34,
36,
37).
Tick bites in susceptible or weakened and immunologically compromised animals may therefore result in one or multiple infections. When introducing wild or domestic ruminants in a new pasture, a farm or country, it is important to consider which disease and which parasite they may introduce, but it is also imperative to consider the danger they incur when facing new parasites and new microbial pathogens.
CONCLUSION
This study identified the presence of
Babesia divergens absent in the USA and French strain of
Anaplasma phagocytophilum in reindeer specimen infested with
Ixodes ricinus and imported from the USA. This concludes that tick-borne pathogens can be widely dispersed via vector transmission. The importation and transportation of wild animals is a significant risk factor.
Blood smears and molecular identification methods are effective in detection of tick-borne pathogens in suspect wild ruminants and should be routinely performed and updated.
CONFLICT OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The authors cordially thank C. Ferlat, DVM, who referred these cases. This work is part of the research project about haemobacteria in wild and domestic ruminants performed in the Interaction of Host and Pathogen Agents (IHAP) unit. It is also part of LR’s residency program (European College of Bovine Health Management-ECBHM).
AUTHORS’ CONTRIBUTIONS
LR performed the clinical examination, necropsies, blood smears, blood analysis and partially the PCR analysis. RPM performed the morphological diagnosis from the blood smears and part of PCR and blast analysis. Both authors contributed equally in writing this manuscript.

 10.2478/macvetrev-2020-0023
10.2478/macvetrev-2020-0023



