Twinning induction of single-bearing Noemi ewes is an important avenue to maximize the economic feasibility of sheep production. Sixty Noemi ewes were used and randomly assigned to six treatment groups (n=10/group).Two sources of FSH [i.e., porcine (P) vs. human (H)] were given as a single dose or in six doses. The control 1 group was given a single dose of saline (C1), while the control 2 group was given six doses of saline (C6). Ewes in group 3 (P1) were given a single dose of p-FSH, in group 4 six doses of p-FSH (P6), in group 5 a single dose of h-FSH (H1), and in group 6 six doses of h-FSH (H6). The ewes were inserted with CIDR for 10 days with FSH given on day 8. A fertile ram was used at the onset of estrus. Blood samples were collected for hormone analyses. The time between CIDR removal and onset of estrus (63, 38 and 26 hrs. in C, P, and H, respectively) was shortened by FSH administration. FSH increased the incidence of twinning, however single dose resulted in more stillbirths and mortalities. The neonatal survival rate decreased in the P1 (40%) compared to the P6 (65%) treatments. Both sources of FSH raised progesterone and estradiol 17-β compared to the controls. Contrariwise, both h- and p-FSH reduced T4; however, h-but not p-FSH raised T3. In conclusion, using rh-FSH at six descending doses of a total 180 IU in Noemi ewes produced two viable neonates. Moreover, the exogenous FSH raised the sex hormones and T3 in the ewes.
Animals
Initially, 60 healthy Noemi ewes of at least 10 months of age (BW 30±3.5 kg) were selected for the study. The ewes were fed a commercial pellet concentrate (15% crude protein) of soya bean, corn, and barley at a rate of 0.35-0.50 kg/ewe/day. Additionally, alfalfa hay, fresh water and mineral blocks were freely available. The study was conducted between January and October 2017 at the Agriculture Experimental Station of the College of Agriculture and Veterinary Medicine, Qassim University, KSA. Animals were handled in accordance with the Qassim University Deanship for Scientific Research Ethical Guidelines for the use and care of animals.
Experimental feeding regimen
Ewes were offered a formulated pellets diet (Saudi Silos Co.) containing 15% crude protein and 65% total digestible nutrients (TDN). For the first 50 days of pregnancy, each ewe was offered 350 g of the pellets in addition to an oral supplement of 75 mg/kg BW of L-Arginine HCL. Thereafter, during the second 50 days of pregnancy, 400 g pellets/head per day were given. However, during the last 50 days of pregnancy, each pregnant ewe was given 500 g of pellets and orally supplemented with 50 mL of date molasses as a source of simple sugar. Clean tap water and mineral blocks were freely offered throughout the experimental period.
Experimental outline
Controlled internal drug release devices (CIDR® device; InterAg, New Zealand) were inserted in the ewe’s vaginas and remained for 10 days. This device is pre-coated with 0.3 g of progestagen in a silicon rubber elastomer. After 8 days of the CIDR insertion, the ewes were randomly assigned into six treatment groups as follows:
Treatments I and II (Controls) - Twenty ewes were assigned into two groups (10 ewes each). Group 1 (C1) ewes were injected (i.m.) with physiological saline solution (0.9% NaCl) in a single dose at 8.00 a.m. on day (D) 8 of the CIDR insertion. Group 2 (C6) ewes were injected (i.m.) with the same amount of physiological saline solution, divided in six doses, given twice daily (at 8.00 a.m. and 8.00 p.m.) on D 8, 9, and 10 of the CIDR insertion. The CIDR was removed on D10 (a.m.). Twenty-four hours after CIDR removal, all ewes in the experiment were presented to fertile rams for natural mating. Ewes in estrus were allowed to be mated twice at 12 h intervals by the same fertile ram. Ewes not showing signs of estrus received a second CIDR insert and the same treatment was repeated.
Treatments III and IV (h-FSH) - In treatments III and IV, 20 ewes were equally divided into two groups; 10 ewes were treated with recombinant human FSH (GONAL-f, Follitropin alpha, Serono, Bari, Italy) as a single dose (H1) and 10 ewes were given the same amount, divided into six doses (H6). In group 3 (H1, n=10) ewes were intramuscularly injected with a total dose of 180 IU h-FSH on D 8 (a.m.). On D 10 (a.m.) CIDR was removed and 180 IU hCG (Pregnyl, Merck Sharp & Dohme) was injected (i.m.). In group 4 (H6, n=10) ewes were administered intramuscularly with a recombinant human follicle stimulating hormone, twice daily (a.m. and p.m.), in descending doses of 40/40 (D 8), 30/30 (D 9) and 20/20 IU (D 10). On D 10 concomitantly with the fifth dose of FSH, a total dose of 180 IU hCG was intramuscularly injected and the CIDR was removed (D 10, a.m.).
Treatments V and VI (p-FSH) - In treatments V and VI, 20 ewes were equally divided into two groups. P1 received a single dose and P6 multiple doses of porcine FSH. Group 5 (P1) ewes were administrated intramuscularly with a dose of 133.33 mg p-FSH (equivalent to 180 IU h-FSH) on day 8 (a.m.). Following CIDR removal, an equivalent dose of hCG was intramuscularly injected on day 10 (a.m.). In group 6 (P6), ewes were administered intramuscularly with porcine follicle stimulating hormone (p-FSH; Folltropin-VEmballage double, Vetoquinol, Canada), twice daily in descending doses of 26.66/26.66 (D 8), 22.85/22.85 (D 9), and 17.15/17.15 mg (D 10). On D 10 concomitantly with the fifth dose, an equivalent dose of 180 IU hCG was intramuscularly injected and the CIDR was removed on D10 (a.m.).
Maternal and neonatal traits
The time between CIDR removal and visible estrus onset was recorded in hours. Pregnancy was diagnosed 40 days post-mating by the aid of the ultrasound machine (ALOKA SSD 500; ALOKA, Japan) fitted with 5 MHz linear array trans-rectal transducer and was confirmed by the abdominal diagnosis using the same transducer.
The following parameters were recorded:
Ewes displaying estrus (percentile): Number of ewes exhibiting estrus/number of ewes under treatment × 100
Pregnancy rate: Number of pregnant ewes/number of ewes mated × 100
Lambing rate: Number of ewes lambing/number of ewes mated × 100
Single-lambing rate: Number of single births/total number of births × 100
Twinning rate: Number of twin births/total number of births × 100
Survival at birth: Number of live births/total number of births
Survival at weaning: Number of weaned lambs/total number of lambs born
Blood sampling and serum harvesting
Blood sampling for hormone analysis was performed by jugular venipuncture (anticoagulated blood) from five ewes in each group, before the CIDR insertion, at the CIDR removal, during estrus, and once a week continuously onward until lambing. If one or more of the selected ewes did not exhibit estrus signs, other subjects of the same group were sampled instead. Plasma was harvested after centrifugation at 1300 × g for 15 minutes in a cold condition (5 °C) and stored in a frozen state (-20 °C) until being analyzed.
Hormone determinations
Tri-iodothyronine (T3) determination Following the method of Bartalena (9) and using a commercial ELISA 96-well kit (Human Gesellschaft, Germany) by the competitive method, plasma and standard samples were measured according to the procedure instructions. The intraassay coefficient of variation (C.V) was 6.8%. The standard levels tested were: 0, 0.5, 1.0, 2.5, 5.0 and 7.5 ng/ml.
Total thyroxin (T4) determination
According to the method of Bartalena (9) and applying the competitive ELISA 96-well method (Human Gesellschaft, Germany), plasma and standard samples were measured following the manufacturer’s instructions. The intra-assay coefficient of variation (C.V) was 7.3%. The standard levels tested were: 0, 2, 5, 10, 15 and 25 μg/dL.
Progesterone (P4) determination
Plasma P4 was determined according to the method of Simersky et al. (10) using a commercial ELISA 96-well kit (Human Gesellschaft, Germany). The procedure was applied according to the manufacturer’s guideline. The standard levels tested were: 0, 0.30, 1.25, 2.50, 5.00, 15.00 and 40.00 ng/mL. The intra-assay coefficient of variation (C.V) was 5.6%.
Estradiol-17ß (E2) determination
Plasma E2 was determined following the method of Ratcliff et al. (11) using a commercial ELISA 96-well kit (Human Gesellschaft, Germany). The procedure for quantifying E2 was performed according to the steps provided by the manufacturer. The standard levels tested were: 0, 25, 100, 250, 500, 1000 and 2000 pg/mL. The intraassay C.V was 6.9% as all samples were analyzed in one assay.
Statistical analysis
Estrus data, time from CIDR removal to onset of estrus and neonatal traits were analyzed by Chi square test. Hormone data were analyzed by the least square analysis of variance for repeated measures using SAS software package (12). The model of statistical analysis used was as follow:
Yijk= μ + Si+ Dj + SiDj + eijk
Yijk= the observation taken on the Kth individual
μ = overall mean
Si= effect of FSH source
Dj= effect of number of FSH injections
SiDj = interaction between source and number of FSH injections
eijk = random error assumed to be in dependent normally distributed with mean = 0 and variance = σ2
Differences between the treatment means were tested by the Duncan's Multiple Range Test (DMRT, 13). Significant differences were considered at P<0.05.
RESULTS
Estrus, follicular dynamics, pregnancy and neonatal traits
As shown in Table 1, the administration of FSH raised the estrus display from 50% in the controls to 90 and 95% in P- and H-treated ewes, respectively. Evidently, giving FSH of either source as one dose improved the estrus exhibition (100%) than when FSH was given in 6 split doses (85%). The duration between CIDR removal and the onset of estrus was shortened (P<0.05) by FSH administration (i.e., 63, 38, and 26 hrs. in C, P and H, respectively). Giving p-FSH as a single dose reduced this duration by about 25%. However, giving p-FSH as a series of six doses reduced (P<0.05) this duration by about 55% compared with controls. Moreover, h-FSH considerably shortened this period by about 59% compared to the controls. At the time of estrus, FSH increased the number of dominant follicles compared with control with insignificant differences between the two sources of FSH or even between the two administration protocols. A similar trend was found on the number of corpora lutea on both ovaries at day 40 post-breeding (Table 1).
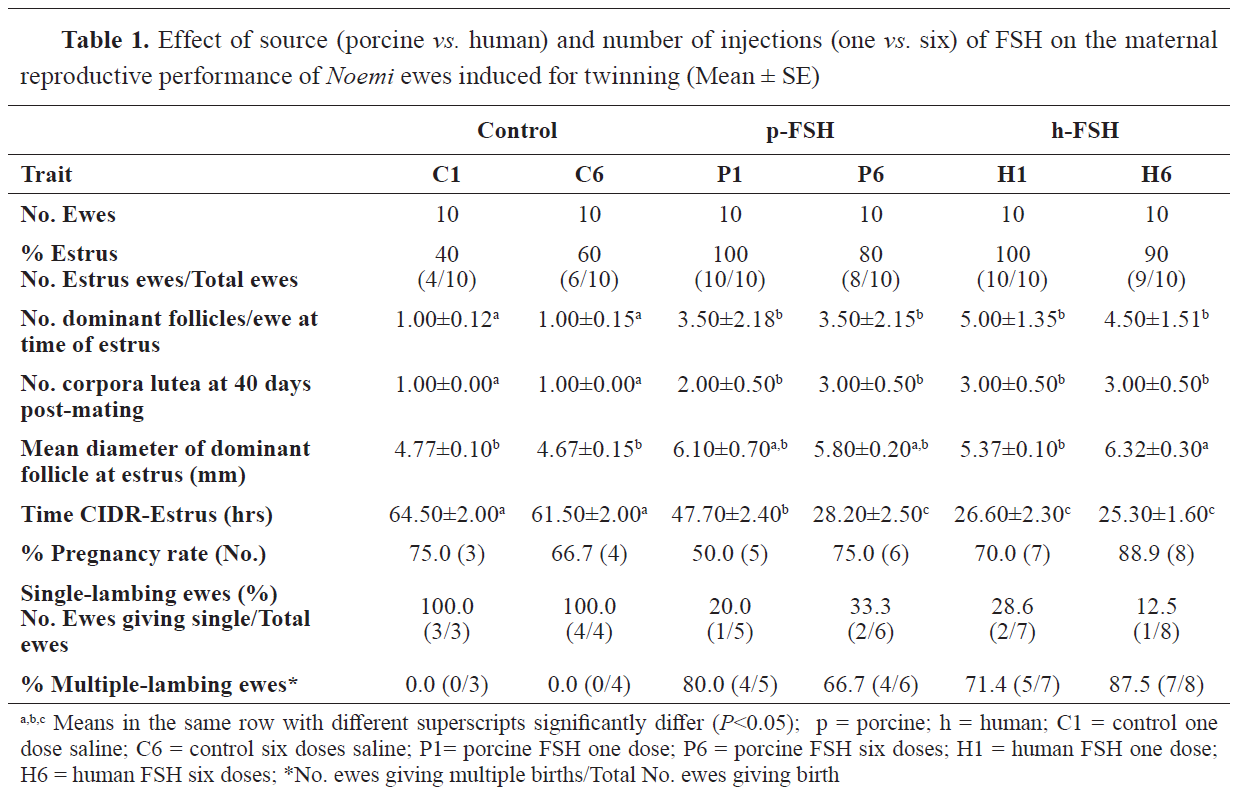
The sole treatment produced larger dominant follicles in H6, however, other treatments resulted in similar follicle size as the control. FSH administration caused an increase in pregnancy rates from 35% in controls, 55% in p-FSH, and 75% in h-FSH-treated ewes. The percent of pregnant ewes within a group giving birth to multiple (>1 lamb) progeny was 0, 72.7, and 80.0%, in the C, P, and H groups, respectively. Survival rates at birth and weaning decreased (
P<0.05) with FSH treatment, showing a greater reduction when FSH of either source was given as a single dose. However, giving FSH in a series of descending doses enhanced the survival rates at birth and weaning, although they were still lower than those in the control (single- bearing) ewes.
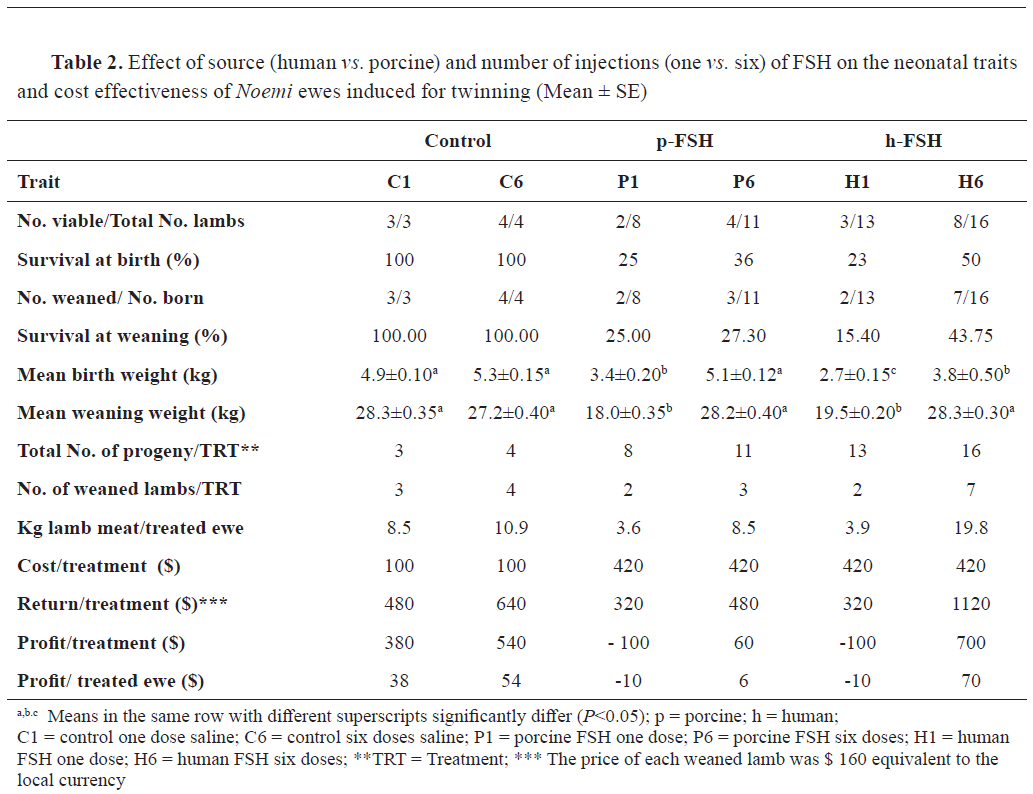
Table 2 presents the data of the neonates. Mean birth weights were 5.15, 3.40, 5.10, 2.70, and 3.80 kg in the C, P1, P6, H1, and H6 ewes, respectively. The birth weight was lowered with FSH treatment, except in the case of P6. The reductions in birth weights were 16 and 36% respectively in P- and H- compared to C-lambs. On the other hand, mean weaning weight at 3 months was lower in P1 and H1 (mean weight of 18.75 kg) compared to P6 and H6 (mean weight of 28.15 kg) and C1 and C6 (mean weight of 27.75 kg). The total outcome of lamb weights per ewe under treatment was highest (
P<0.05) in the H6 ewes. The cost-effect study revealed that the best feasible treatment was H6, resulting in a $70 profit per each treated head. In contrast, giving either p- or h-FSH in one dose resulted in losses of $10 per each treated ewe due to the high mortalities.
Hormone profiles
Tri-iodothyronine (T3) concentration and profile
Human FSH at one dose (H1) showed the highest (P<0.05) level of T3 in blood (1.98 ng/mL), However, the other treatments did not differ significantly from the control (1.71, 1.79, 1.69, and 1.82 ng/mL in C, H6, P1, and P6, respectively).
As illustrated in Fig. 1a, normal levels of T3 in the control ewes fluctuated between ~1 and 3 ng/mL, having a sharp decline at 16-17 weeks of gestation. Thereafter, the T3 levels rose with another peak at the time of parturition.
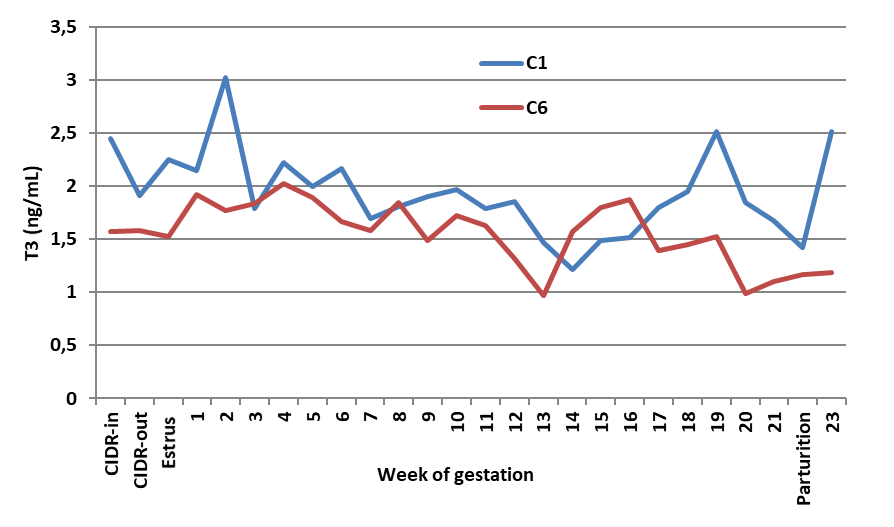 Figure 1a.
Figure 1a. Effect of saline given as one or six doses on blood T3 of Noemi ewes (C1 = one dose; C6 = six doses)
Administration of FSH of either human (
Fig. 1b) or porcine (
Fig. 1c) source increased the degree of T3 f luctuation throughout gestation, showing a peak at parturition. Statistically, T3 levels increased (
P<0.05) only in H1 ewes compared with the controls, however, other treatments did not affect T3 concentration. Meanwhile, regardless of the source, a single dose of FSH elevated T3 levels (
P<0.05), whereas six divided doses did not change T3 levels compared to the controls.
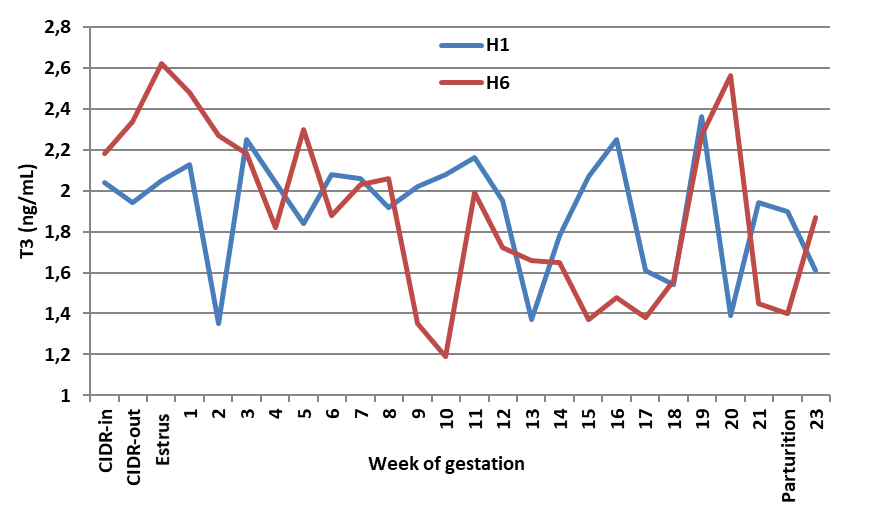 Figure 1b.
Figure 1b. Effect of human FSH given as one or six doses on blood T3 of Noemi ewes induced for twinning (H1 = one dose h-FSH; H6 = six doses h-FSH)
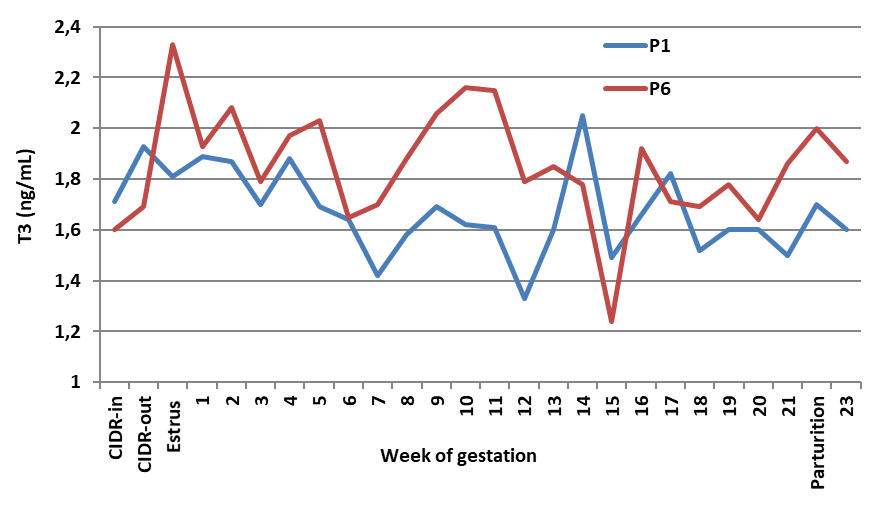 Figure 1c.
Figure 1c. Effect of porcine FSH given as one or six doses on blood T3 of Noemi ewes induced for twinning (P1 = one dose p-FSH; P6 = six doses p-FSH)
Thyroxin (T4) concentration and profile
Fig. 2a shows the pattern of blood T4 in the control ewes. At parturition, the level of T4 ranges between 9.5 and 14.0 μg/dL (i.e., equivalent to 95 ‒140 ng/mL). On the other hand, administration of FSH of either source significantly (P<0.05) reduced T4 levels throughout gestation, and thus, T4 levels at delivery were lower compared to the counterpart control ewes.
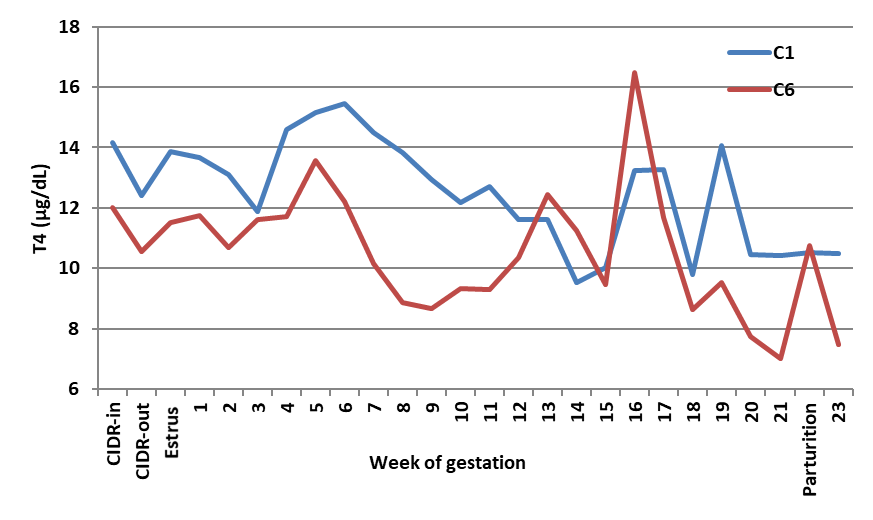 Figure 2a.
Figure 2a. Effect of saline given as one or six doses on blood T4 of Noemi ewes (C1 = one dose saline; C6 = six doses saline)
Mean reduction in blood T4 due to FSH injection was 38% in H- (
Fig. 2b) and 45% (
Fig. 2c) in P-ewes. The best treatment resulting in the highest number of viable lambs was H6, which maintained normal levels of T3 and reduced (P<0.05) mean levels of 4, but maintained T4 levels at parturition, similar to the trend observed in the control ewes.
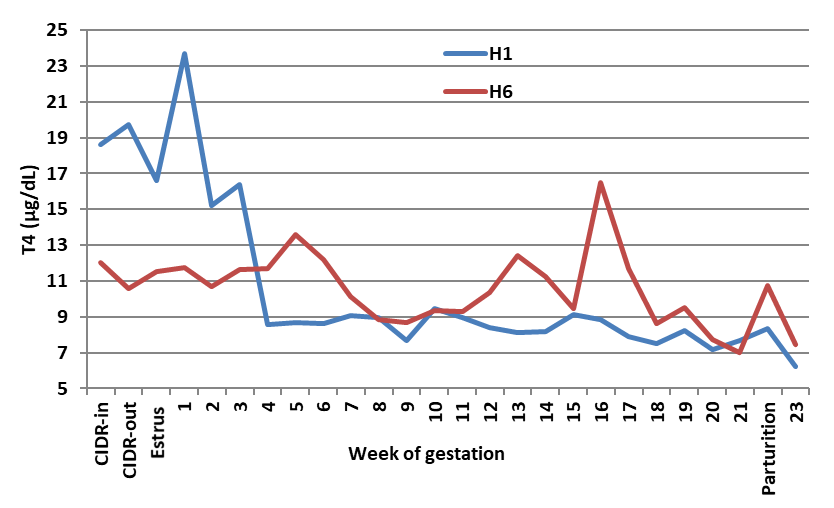 Figure 2b.
Figure 2b. Effect of human FSH given as one or six doses on blood T4 of Noemi ewes induced for twinning (H1 = one dose of h-FSH; H6 = six doses of h-FSH)
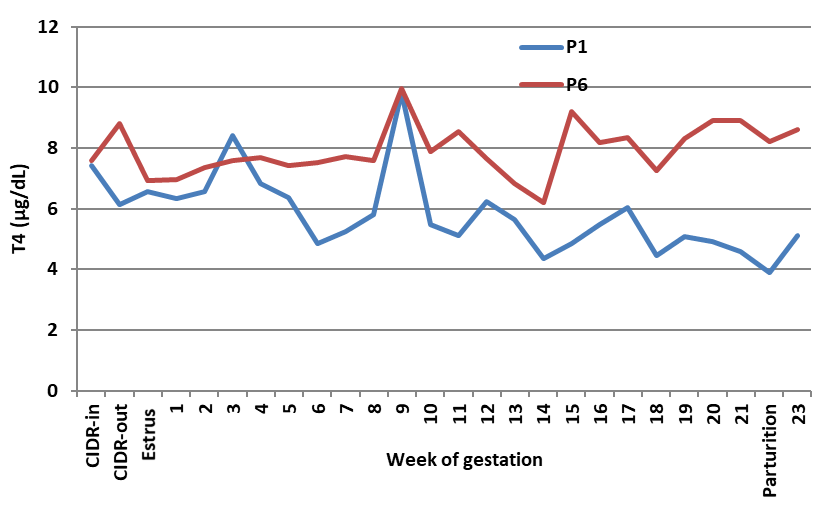 Figure 2c.
Figure 2c. Effect of porcine FSH given as one or six doses on blood T4 of Noemi ewes induced for twinning (P1 = one dose p-FSH; P6 = six doses p-FSH)
Progesterone (P4) concentration and profile Administration of FSH of either source caused an increase (P<0.05) in peripheral P4 concentrations in ewes (
Fig. 3).
 Figure 3.
Figure 3. Effect of source and series of FSH injections on mean blood progesterone of CIDR implanted - Noemi ewes induced for twinning during gestation and parturition (C = saline; H1 = one dose h-FSH; H6 = six doses h-FSH; P1 = one dose p-FSH; P6 = six doses p-FSH)
The P4 increase was more pronounced in the ewes given porcine than in their counterparts given human FSH (3.72, 5.18, and 9.18 ng/mL in C, H, and P, respectively). Moreover, giving FSH as a series of injections increased blood concentrations of P4 higher than single dose injections (3.77, 6.59, and 8.47 ng/mL in C, single-dose, and six doses, respectively).
In control pregnant ewes, the peripheral concentration of P4 declined to near zero at the onset of estrus and rose gradually reaching a peak level of 6-6.5 ng/ml, then declined to near 1 ng/mL at delivery. On the other hand, giving human or porcine FSH maintained the highest concentrations of P4 in a plateau for a longer time throughout pregnancy, with the fact that porcine FSH maintained higher (P<0.05) P4 levels than human FSH and control.
Estradiol (E2) concentration and profile
As illustrated in
Fig. 4, the sole treatment that elevated (P<0.05) peripheral E2 levels was H6. There were mathematical, but no statistical differences (P>0.05) due to other treatments on E2 levels with non-significant effects.
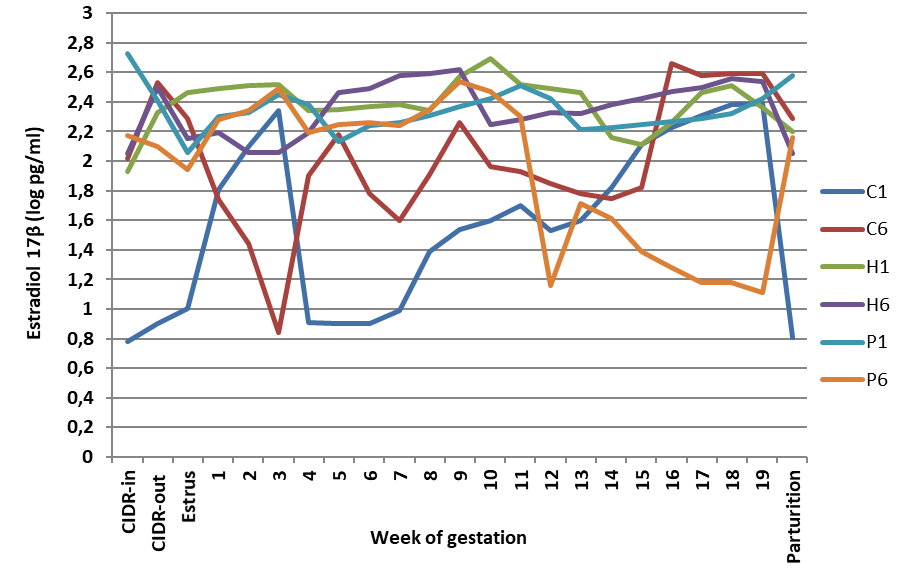
In the control pregnant ewes (i.e., those given saline), there were two peaks of estradiol. The first peak appeared on D 21-35 and the second peak on D 78-89 of gestation. Additionally, at the time of parturition, blood E2 concentrations increased to reach 250-450 pg/mL. In contrast, the administration of porcine or human FSH resulted in minimizing blood E2 to nearly zero. Treatment with either source of FSH enhanced E2 production with the utmost enhancement observed in H6 treatment.
DISCUSSION
Sheep breeds differ in their prolificacy according to their genetic composition. The indigenous breeds in Saudi Arabia and the Gulf region lack the multiple-birth genes (
2). Moreover, based on the fact that the reproductive traits are of low heritability, a search was instigated for an environmental avenue to enhance the productivity of these farm animals (
14). It is well known that FSH molecules bind to granulosa cells (GCs) initiating the kinetic reactions (
15). The administration of the cumulative dose of FSH (i.e., 180 IU human FSH or 133.33 mg porcine FSH) in one injection in the current study increased the incidence of estrus among ewes. However, splitting the total dose into six descending doses of FSH may have gradually bound it to the GCs in a timely manner, evoking the hypothalamo-pituitary axis to release the internal gonadotropin hormones, sequentially leading to the release of estrogens (
16).
The interval between CIDR removal and onset of estrus was shortened by FSH injection with either mode, paralleling the finding in cattle by Larfi et al. (
17) who reported a reduction of 11-14 h in this interval due to exogenous FSH injection. The FSH injection in the current study shortened this interval by 24 to 36 h, which facilitated the advent of estrus in a shorter time enabling better management. A similar observation was reported in Awassi sheep (
18).
The survival rate at birth diminished with the single FSH injection compared to the series of injections. Evidently, there appears to have been simultaneous ovulation and subsequent fertilization in the P1 and H1 ewes, resulting in congestion of the implanted fetuses competing for space and available nutrients in the uterine environment. This phenomenon caused what is known as intrauterine growth restriction (IUGR), resulting in several cases of stillborn lambs. Gootwine et al. (
19) studied the effect of litter size in sheep on the individual health and wellbeing of the neonates and they reported that lambs born of ewes carrying multiple fetuses had relatively small placentas with fewer cotyledons and lower birth weights. Administration of FSH in descending doses over a 72-h period might allow sufficient time for each embryo to get implanted in the uterine endometrial membranes. Thus, the finding of the current study showing the lowest survival rate at birth in the ewes given a single dose of either source of FSH (23 and 25% in P1 and H1, respectively) corresponds with the idea of the occurrence of IUGR. It has been reported that ewes carrying twins or multiple fetuses suffer IUGR more than those carrying single fetuses (
19). In their review on human IUGR, Sharma et al. (
20) attributed the high fetal morbidity and mortality in such cases to the incidence of perinatal asphyxia, hypothermia, and polycythemia. Nonetheless, the placenta size was found to be smaller in multiplebearing ewes than in single-bearing ewes and fewer cotyledons were available for each fetus. Placental insufficiency causing chronic hypoxia has been reported as one of the main causes of IUGR (
20). Following the similarities between sheep and human pregnancy, especially in singleton pregnancies, IUGR has similar characteristics in both species (
21,
22). Hypoxia and lower availability of nutrients may impede fetal development and ultimately cause death of the conceptus, as well as leading to IUGR and reduction of birth weight and postnatal growth (
23). This observation was evident in the current study as the shares of placenta for each fetus in the twin or multiple-fetus pregnancies was lower (data not shown).
The best treatment resulting in acceptable survival at weaning was H6, which provided the best lamb outcome at weaning. Placental weight and fetal weight are not well-correlated during early gestation; however, at late gestation and near delivery, they are highly correlated (
24,
25). In the current study, the drenching of ewes with L-arginine for the first 50 days of gestation may have sustained the excess release of nitrous oxide (NO), leading to increase of placental angiogenesis and blood flow (
26). Zeitoun et al. (
27) stated that supplementing pregnant ewes with low doses (75 mg/kg BW/56 days) of L-arginine at the early stage of gestation increased lamb birth weight and survival, and improved maternal health. Furthermore, giving ewes daily doses of date molasses during late pregnancy enhanced milk secretion to a sufficient degree for the needs of twin lambs. The resultant similar weaning weight of each individual twinlamb to the single lambs born of the control ewes confirms that the twin-bearing ewes have achieved a positive energy balance. Abdelsalam et al. (
28) stated that giving pregnant Najdi ewes oral palm date syrup during last eight weeks of pregnancy increased birth weights by 85% over the control births.
Evidently based on the purity of the molecules of the recombinant human FSH aimed at treating sub-fertility in women, its effects on the ovarian response and estradiol 17β production surpassed the porcine FSH. The porcine pituitary extract of FSH seemed to be less pure than the h-FSH and to be contaminated by the presence of LH molecules. This observation is clear in the higher estradiol 17β production in the h-FSH-treated ewes. On the other hand, the porcine FSH induced greater elevations in progesterone than the h-FSH. Estradiol production is a response to the binding of the FSH molecules to the receptors in the granulosa, converting thecalderived androgens into estrogens by the cells of the ovarian mature follicles (
29,
30). The purity of pituitary FSH depends on the purification process; however, the specificity of the recombinant FSH is more accurate (The Practice Committee of the American Society for Reproductive Medicine, Birmingham) (
31). It has been stated that the advent of recombinant DNA technology paved the way for the production of recombinant FSH preparations. The manufacturing of recombinant preparations is achieved by inserting the genes encoding for the alpha and beta subunits of FSH into expression vectors that are transfected into Chinese hamster ovary cell lines (
32).
Regarding thyroid hormones (T3 and T4), the highest level of T3 in the blood was found in ewes given a single dose of human FSH. There are several studies in the literature reporting on the relationship between thyroid hormones and reproduction (
33). It has been previously documented that T3 and T4 directly change the steroidogenic capacity of porcine and human granulosa cells. Hayashi et al. (
34) and Maruo et al. (
35) observed that T4 stimulated FSH-induced E2 production in porcine granulosa cells. Moreover, Wakim et al. (
36,
37) reported increases of E2 and P4 in human granulosa cells due to T4 supplementation. The reduction in T4 levels resulting from the FSH was referred to by Spicer et al. (38) in cattle; however, these two hormones (T3 and T4) promoted the production of P4 and E2 through a multi-hormonal regulation of follicular steroidogenesis.
CONCLUSION
Administration of FSH of either source as one cumulative dose has shown hormonal imbalances and spontaneous ovulations resulting in stillborn or mummified fetuses. However, human FSH resulted in better ovarian response and proper sequential ovulations and proper uterine milieu for fetal implantation. Thus, in order to optimize a protocol for obtaining viable twin lambs of single-bearing ewes, it is recommended that a recombinant human FSH to be used due to its specificity and purity, it should be given in six descending doses to achieve a total of 180 IU, in conjunction with CIDR andan equivalent dose of hCG. Furthermore, a source of simple carbohydrates should be supplemented during the last trimester and L-arginine at low dose must be provided during the first trimester. The cost-effective outcome resulting from application of such a protocol is economically feasible for sheep owners. For the most reliable results, this protocol should be applied on a larger number of animals and during different seasons of the year.
CONFLICTS OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial support of the Qassim University Scientific Research Deanship (QU-SRD) and thank them for sponsoring this research. In addition, we acknowledge the help of the animal research station crew.
AUTHORS’ CONTRIBUTIONS
MMZ proposed the experimental design, supervised the field tasks, analyzed the hormones, data curation, and wrote the final version of the manuscript. MAA conducted the hormone administration, ultrasound diagnoses, blood collection, animal mating, and keeping data of ewe's delivery. AOE has conducted the field treatments, helped in the laboratory analyses and wrote the first draft of the manuscript.

 10.2478/macvetrev-2020-0024
10.2478/macvetrev-2020-0024









