The polyetiological syndrome of disseminated intravascular coagulation (DIC) is characterized by changes in patients’ hemostasis. The aim of the current research was to elucidate the main factors for the development of DIC syndrome during canine babesiosis, and to assess their correlation level. Dogs included in this study were of various breeds and sex, weighing 10-40 kg and aged 2-7 years. They were separated in two groups (n=50) according to their diagnosis to babesiosis. Oscillometry (blood pressure, pulse rate), vascular-platelet hemostasis, coagulogram, hematological, biochemical (fibrinogen, fibrin degradation product, soluble fibrin-monomer complex) and hemodynamic (circulating blood volume) assessment methods were used. The group of dogs positive on Babesia spp., had clear manifestation of DIC with 5-7% of the erythrocyte population being affected. DIC was manifested by a significant increase in soluble fibrin-monomer complex and fibrin degradation product (p<0.001), hypofibrinogenemia (p<0.001), thrombocytopenia (p<0.001), and an increase in indicators of spontaneous aggregation ability of platelets and red blood cells (p<0.001). Significant hemodynamic disorders were observed: a decrease in circulating blood volume, circulating erythrocytes volume (p<0.05), specific circulating blood volume and hematocrit value (p<0.001). The average blood pressure was reduced (p<0.001), and the Allgöwer’s shock index was increased 2 times (p<0.05). A shock of II degree (medium, subcompensated) was confirmed. Therefore, it can be concluded that acute spontaneous dogs’ babesiosis can be characterized by the occurrence of DIC in a consumption coagulopathy form, and shock of II degree. This condition renders the patients for emergency admission.
Canine babesiosis is a common tick-transmitted disease of dogs worldwide. The causative agents are intracellular apicomplex hemoparasites
Babesia spp., which are common throughout the world (
1,
2).
Babesia spp. are classified as large
Babesia (
B. canis Piana et Galli-Valerio, 1895,
B. vogeli Reichenow, 1937,
B. rossi Nutall, 1910) and small-
Babesia (
B. gibsoni Patton, 1910,
B. conradae sp. nov.,
B. vulpes Baneth, Florin-Christensen, Cardoso & Schnittger 2015) (
3,
4).
The predominant babesiosis pathogen in certain geographical regions may be correlated with animal movement, tick’s dispersion, etc.
B.canis predominantly occurs in Europe,
B. rossi in Affrica,
B. vogeli is globally widespread, whereas small
Babesia is predominant in Asia. Various
Babesia spp. are characterized by different virulence (
3,
4). Ixodid ticks of genus
Dermacentor Koch, 1844,
Rhipicephalus Koch, 1844,
Ixodes Latreille, 1795 (
5) are regarded as vectors for
Babesia spp.
Babesiosis is a bloodborne disease, which may result in severe clinical condition. Hemolysis leads to hypoxia in various organs resulting in hemodynamic, hemorheological, and microcirculatory disorders (
6,
7,
8,
9,
10). The most common outcome of these pathological manifestations is the disseminated intravascular coagulation (DIC) syndrome. The underlying pathophysiological mechanisms are the intravascular release of blood coagulation activators, platelet aggregation, thrombin formation, plasma enzyme systems activation and depletion, and formation of numerous intravascular micro-clots and cell-aggregates which cause organ-microcirculation disruption. These changes lead to development of thrombohemorrhages, hypoxia, acidosis, organ parenchymal dystrophy and dysfunction, as well as intoxication of the body with protein decay products and other metabolites (
9,
10,
11). DIC-syndrome has progressive clinical manifestation which may lead to animal death. Hence, a positive diagnosis of babesiosis in dogs should be immediately followed by DIC assessment and appropriate treatment.
The aim of the current research was to elucidate the main factors for the development of DIC syndrome during canine babesiosis, and to assess their correlation level.
MATERIAL AND METHODS
The studies were performed in the period of 2017–2018, on the premises of the Veterinary Medicine Clinic of Zhytomyr National Agroecological University and the Laboratory of Parasitology of the Faculty of Veterinary Medicine.
Ethics Committee approval was received for this study from the Ethics Committee of the Zhytomyr National Agroecoligical University (Approval number: 2017/07).
Sampling
The study was conducted on 100 dogs. The experimental and control groups were formed according to the principle of pair-analoges, 50 animals each.
Dogs were from different breeds and sex, aged in the range of 2–7 years, and body weight in the range of 10–40 kg. The experimental group included dogs with acute spontaneous babesiosis. The control group included clinically healthy dogs (the animals had no clinical signs of any diseases; there was no history of contact with ixodid ticks; no pathogens were detected in the stained blood smears; hematological parameters were within the normal reference range).
Clinical and microscopic studies
Clinical studies were performed according to the general methods of clinical research.
The basic diagnostic procedure was the detection of
Babesia spp. in thin blood smears stained with Giemsa (
12).
The causative agent was situated in the middle of the erythrocyte. Agents were pear-shaped, exceeding the radius of the red blood cell and located at an acute angle to each other.
The causative agent has been identified as a large
Babesia spp. (
13,
14). Specific typing was not performed.
The average intensity of parasitemia was 5–7% affected erythrocytes.
Hematological and studies of vascular platelet haemostasis
Blood sampling was performed from
V. saphena antebrachii. A solution of sodium citrate 3.8% in a ratio of 1:10 was used as an anticoagulant (
12,
15).
The hemoglobin content, the number of formed elements (red blood cells, white blood cells, and platelets), erythrocyte sedimentation rate, and hematocrit value (Ht) were determined using a Mindray BC-3600 (Mindray Medical Rus Co. Ltd, Russian Federation) hematology analyzer.
The vascular-platelet link of hemostasis was assessed by evaluating the spontaneous aggregation ability of platelets and erythrocytes according to Tarasova (
16).
Assessment of haemorheological parameters
Haemorheological parameters included the determination of circulating blood volume (CBV) and its components, as well as the calculation of specific circulating blood volume (SCBV) (ml/kg) using the T-1824 blue Evans dye dilution method (
17,
18). Blood loss volume (BLV) was determined using the Moore hematocrit method according to the equations provided in
table 1 (
1).
Measurement of blood pressure and its indices
Systole arterial pressure (Sys), diastole arterial pressure (Dia), pulse (P) mean arterial pressure (MAP) and
Allgöwer’s shock index (ASI) (
19,
20) were used as blood pressure indices. PetMap graphic II (CardioCommand, USA) veterinary blood pressure monitor was used. MAP and ASI were calculated according to the equations provided in
Table 1 (
2).
 Assessment of the coagulation unit and fibrinolytic components
Assessment of the coagulation unit and fibrinolytic components
The coagulation link of hemostasis was evaluated using a TS-4000 (HTI, USA) coagulometer. Coagulogram indices were determined according to the time of spontaneous blood coagulation, time of platelet-free plasma recalcification (the time of the coagulation contact phase was determined by the difference of these indicators), prothrombin time (PT) (21), thrombin time (TT), partial thromboplastin time (PTT) (
22), plasma-activated partial thromboplastin time (APTT) (
22), platelet factor 3 availability (by the difference between PTT and APTT), factor XIII activity, plasma tolerance to heparin, and lysis of euglobulin clots (
9,
10).
The fibrinolytic component (blood clot retraction, spontaneous fibrinolysis) was evaluated by measuring the volume of the formed clot, extracted serum and the extracted blood cells following 3 hours after clotting (
16). This measurement was of a qualitative nature (screening test).
Biochemical research
The soluble fibrin-monomer complexes (SFMC) content was quantified with the help of the orthophenanthroline method (
10,
12). Fibrinogen levels were evaluated by Klaus clotting using the coagulometer HumaClot DUO Plus (Human GmbH, Germany) (
12). The content of fibrin degradation product (FDP) was determined by enzyme-linked immunosorbent assay (
10).
Statistical analysis
Statistical processing of the results was carried out using Statistica 13.3 IT Application. We performed multiple variance comparisons using the Fisher distribution (ANOVA). Analysis of variance (ANOVA) was used for determining a statistically significant effect on the factors studied. The reliability of the data was evaluated by the Fisher F-test at a confidence level p<0.05.
RESULTS
The experimental group dogs included in this study had tick infestation, fever, pallor of the mucous membrane with icterus after 3–4 days, hemoglobinuria (60%), tachycardia, tachypnoea, arrhythmia, impaired coordination of movements (35%), and vomiting with the presence of red bile (25%). In 7 dogs (14%), spotted and spotty hemorrhages were observed on the mucous membranes and skin, including hypothermia and a cold perspiration. Death was confirmed in 3 dogs.
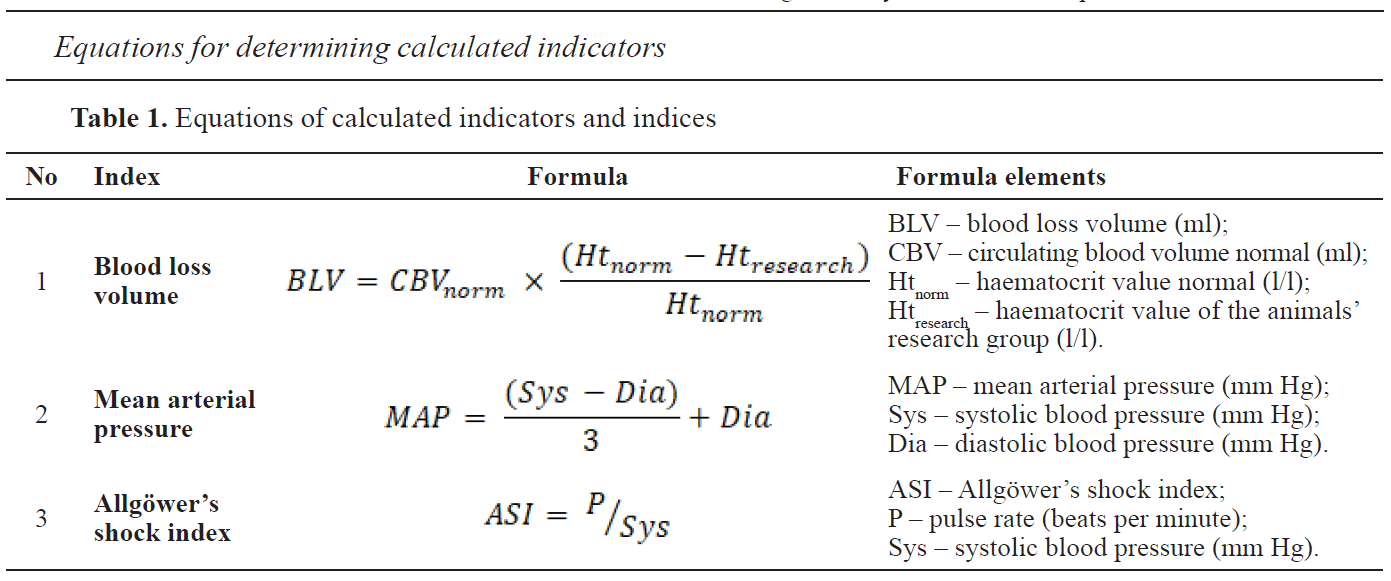
Significant vascular-platelet hemostasis developed in all experimental group dogs: thrombocytopenia and an increase in the spontaneous aggregation ability of platelets and red blood cells (
Table 2).
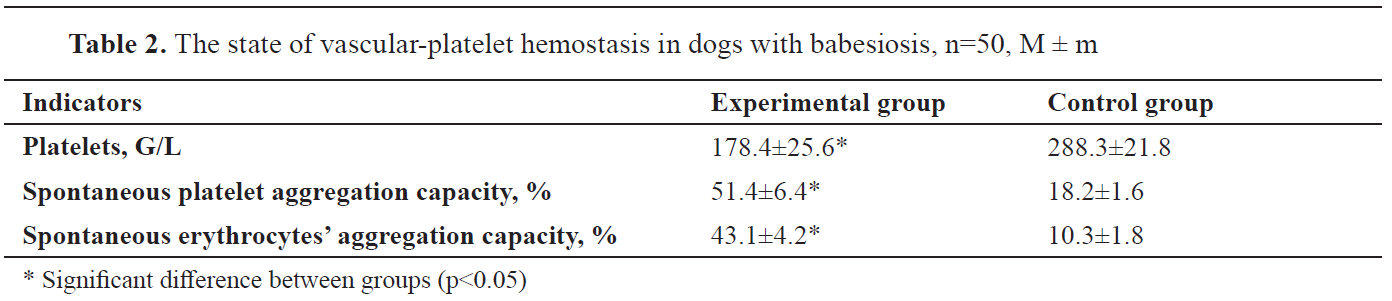
In the experimental group dogs, the hemoglobin content, the number of red blood cells and Ht were reduced, whereas the number of leukocytes and
the erythrocyte sedimentation rate were increased (
Table 3).
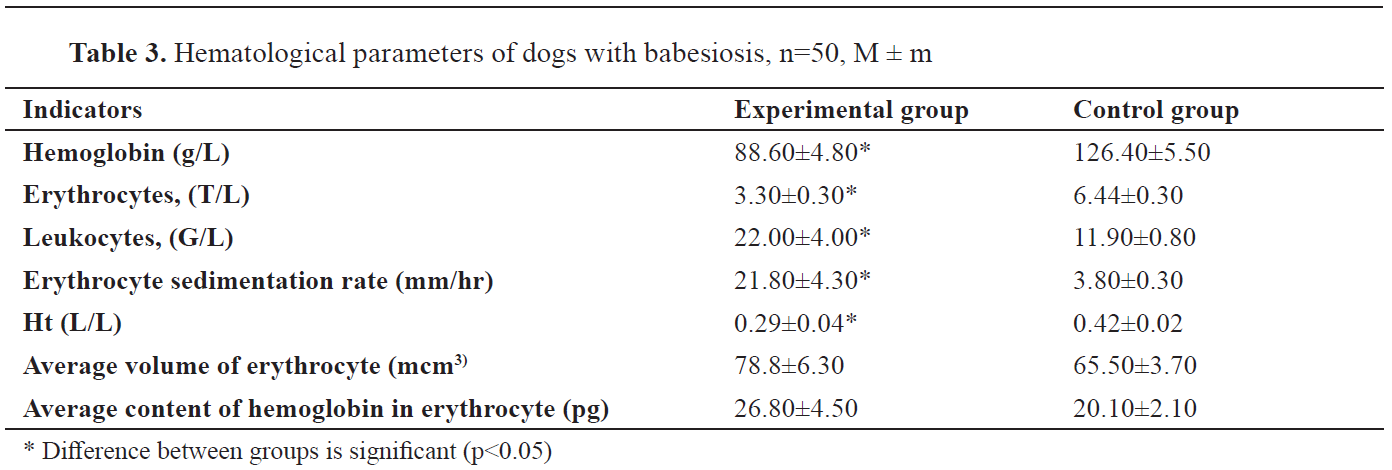
Haemorheological parameters in dogs with babesiosis had significant changes (
Table 4). Indicators of Ht, SCBV, and circulating erythrocytes volume (CEV) were significantly reduced.
Blood loss was detected in experimental group dogs – an average of 1500 ml (31%).
Oscillometric measurements were used for calculating the indices for the hemodynamic state of the animals (
Table 5).

Significant changes were found in the hemostasiologic link of dogs with babesiosis (
Tables 6 and
7).
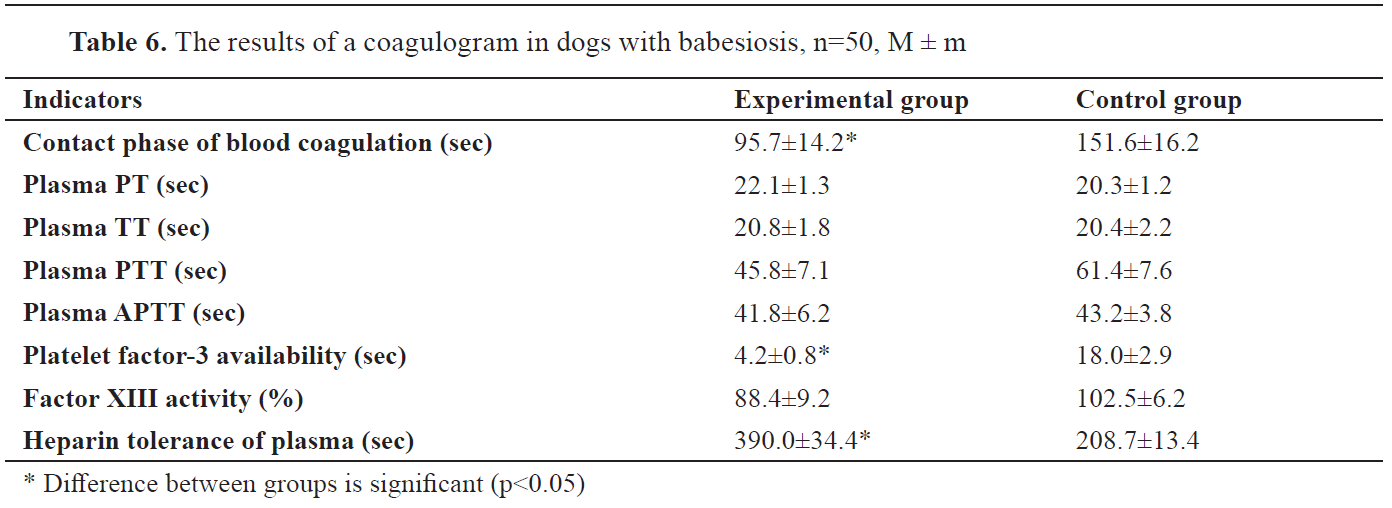
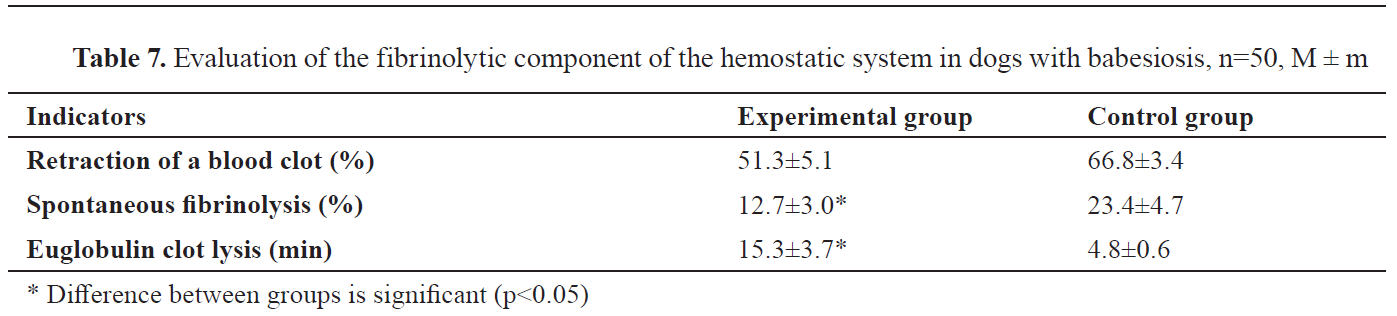
In coagulation tests, multidirectional shifts were determined. Coagulation acceleration was noted in indices of the contact phase, thromboplastin coagulation, platelet factor 3 activity and spontaneous fibrinolysis. Coagulation retardation was manifested in the plasma PT test, factor XIII activity, plasma tolerance to heparin, lysis of euglobulin clots, and retraction of a blood clot.
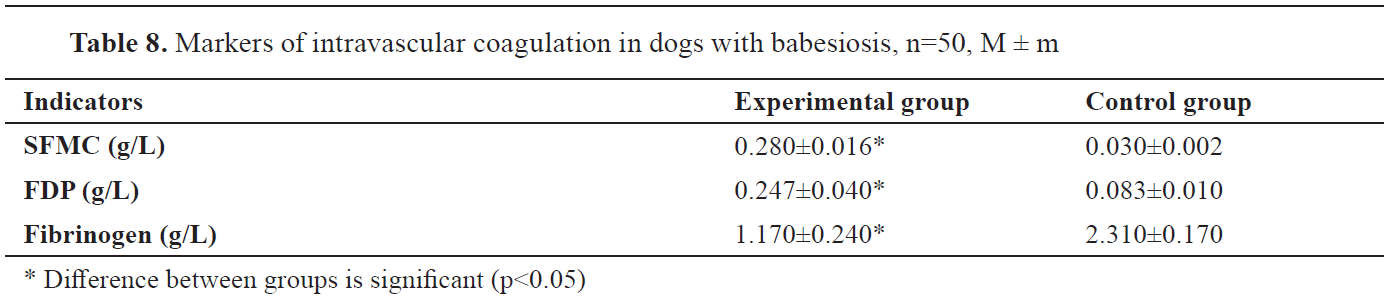
DIC markers were identified and a significant increase in the levels of SFMC and FDP was detected along with hypofibrinogenemia (
Table 8).
DISCUSSION
Acute babesiosis in dogs is frequently accompanied by vital organ dysfunction. The interaction between the body’s immunological response and the pathogen results in intravascular hemolysis, anemia, etc. (
6,
7,
8). Consequently, the DIC syndrome occurs. Initially, generalized coagulation appears due to the activation of external and internal coagulation cascades. This is followed by hypocoagulation which results with hemorrhages (
9,
23).
The correlation between DIC and occurrence of shock has been already demonstrated (
23,
24). Thus, the severity of the animal’s condition positive on babesiosis could be determined by assessing the degree of shock and manifestation of DIC syndrome (
7,
25,
26,
27).
Clinical signs in babesiosis positive dogs are characterized by the presence of normochromic anemia, hemolytic-parenchymal type of jaundice, hepatopathy, nephropathy, cardiovascular failure, and nervous system damage (
2). These underlying pathological manifestations result in multiple organ failure.
Intravascular thrombosis was confirmed in dogs positive on babesiosis by detection of thrombocytopenia, and increased spontaneous aggregation ability of erythrocytes and platelets. Subsequently, aggregates (especially blood clots) entrap red blood cells and platelets resulting in decreased availability in the bloodstream (
9,
10).
The process of blood cells’ aggregation is accompanied by intravascular coagulation. The stimulation of blood coagulation (
9,
28,
29) eventually leads to DIC.
A decrease in CBV was observed in animals positive on babesiosis which was an indication for blood loss. The hemorrhage was characterized by the accumulation of blood in the microcirculatory space, accompanied by edema in the internal organs (
20,
27). The decrease in CBV is a determining factor in the development of shock (
20,
27). In our study, the specific volume of circulating blood was reduced by 1.5 times. According to the Moore equation, the hemorrhage degree was defined as acute-average (31%). Blood pressure and its indices are supporting the diagnosing of shock (
30,
31). In our study, MAP was significantly reduced, and ASI was almost 2 times higher than the control group. This allowed us to classify the shock as II degree (medium, sub-compensated) (
32). A decrease in CBV is closely correlated with a decrease in blood pressure. This causes a decrease in tissue perfusion and leads to extensive cell dysfunction, which is the equivalent of shock (
20,
27). This condition triggers the DIC which exacerbates the overall state of the animal (
9,
20,
26,
27).
The coagulation indicators were multidirectional (
Tables 6 and
7) which was pathognomonic for the DIC syndrome (
9). The plausible explanation for this was the reduced contact phase of the internal coagulation mechanism caused by the activation of factor XII (Hageman) by metabolic products of pathogens, immune complexes, leukocyte factors, etc. (
5,
6,
7,
9,
10). This occurs in combination with vascular-platelet alterations in the form of thrombocytopenia and an increase in the spontaneous aggregation ability of platelets and erythrocytes. This leads to the development of angiopathy and an increase in vascular permeability, which in turn enhances thrombosis (
9).
The platelet-factor 3 (blood thromboplastin) which is released by platelet lysis, is reduced. Blood coagulation is thus enhanced by the internal mechanism of blood thromboplastin activation (
33).
The tendency to lengthen plasma prothrombin time may be associated with the blocking of fibrinogen in SFMC and FDP. An increase in their number was confirmed in our study. This condition confirms the insignificant role of the blood coagulation’s external mechanism in the development of hemostasiopathy in spontaneous canine babesiosis (
33).
The activity of factor XIII coagulation tends to decrease. Its depletion during thrombosis and DIC predisposes failure of polymerization and fibrin stabilization (
9,
28).
In the plasma tolerance test to heparin, significant hypocoagulation changes were found, which trigger the activation of the hemostatic system’s anticoagulant component (
9,
28).
The significant increase in the level of SFMC and FDP in a state of hypofibrinogenemia is an indisputable indication of the DIC syndrome, which develops as a complication in canine babesiosis. According to the DIC pathogenesis (
9,
28,
29), large amounts of fibrinogen are blocked in SFMC and FDP, making it inaccessible to the effects of thrombin.
A significant increase in the level of FDP is combined with a slowdown in euglobulin lysis and a decrease in the spontaneous fibrinolysis. Lysis in blood clots and micro clots of fibrin leads to the entry of a large amount of FDP into the bloodstream. The same blood clots consume the plasminogen and its activator, removing them from the general circulation. Thus, fibrinolytic activity is decreasing (
9,
10).
The formation of SFMC is induced by thrombin. Their mutual increase in blood flow is an indicator of a thrombogenic risk. (
9,
10). Under physiological conditions, a low level of SFMC prevents the immediate coagulation of blood and thus helps to maintain its fluid state. (
28,
29).
The FDP and the SFMC are increasing the blood viscosity, exacerbating rheological disorders. They block a huge amount of fibrinogen, increasing the risk of hypofibrinogenemic bleeding. They also increase the anticoagulant activity of the blood plasma (
9,
28,
29,
34,
35). Thus, in dogs during the acute stage of babesiosis, we confirmed the DIC syndrome at the stage of consumptive coagulopathy. Therefore, the DIC can be considered an integral part of the shock and the leading culprit of the “vicious circle” (
9,
10,
26). This syndrome was the determining cause of death in the three dogs of this study.
The consumptive coagulopathy stage of the DIC syndrome that we confirmed gives multidirectional characteristics of the hemostatic system – thrombogenic risk in the background of anticoagulant activation with the prospect of bleeding. In this case, rheological disorders are clearly expressed, leading to the shock of II degree. Pathogenetically, the development of shock leads to persistent spasm of the capillaries, especially in organs of intensive exchange with the external environment (lungs, kidneys).
Taking the above into account, we consider that in acute spontaneous canine babesiosis DIC syndrome occurs during the consumptive coagulopathy stage. The DIC syndrome is the trigger for shock, which leads to exacerbation of the clinical condition. At the initial examination, the state of the shock was assessed as II degree (medium, sub-compensated). According to the symptomatic classification (
20,
26,
27), this condition is a state of unstable equilibrium and requires use of emergency therapy.
CONCLUSION
The progress of the DIC syndrome is confirmed by the main markers – an increase in the level of soluble fibrin-monomer complexes and fibrin degradation product, hypofibrinogenemia, thrombocytopenia, and an increase in the spontaneous aggregation ability of red blood cells and platelets. The DIC syndrome is a part of the “vicious circle” of shock triggered by the DIC. The shock was determined by the indicators of the circulating blood volume and the mean arterial pressure decreasing, as well as by ascendancy of Allgöwer’s shock index. The abovementioned shock was classified as circulatory, subcompensated, with moderate severity. This defines the disease as urgent and thus it requires immediate use of intensive care for the patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest related to this article.
ACKNOWLEDGEMENTS
This work is the part of a research project of the Department of Parasitology, Veterinary-Sanitary Expertise and Zoohygiene, Zhytomyr National Agroecological University, titled "Research of the canine babesiosis' pathogenesis in terms of pathophysiological complications".
AUTHORS’ CONTRIBUTIONS
OAD gave the idea, concept, research organization; DVF performed laboratory research and evaluation of the results; TIB made the evaluation of the results and correspondence; OAZ performed the laboratory research and literature review; AAA conducted parasitological aspects and methodology; SVR gave interpretation of the results; VPG conducted statistical data processing and interpretation of the results; RVS performed methodology and translation and VSS made the design of the study and translation corrections

 10.2478/macvetrev-2020-0027
10.2478/macvetrev-2020-0027







