We hypothesized that a single dose of PGF2α belatedly injected on day 8 after GnRH-1 in cows receiving a 7-day Ovsynch-56 protocol (GnRH – 7 days – PGF2α – 56h – GnRH – 16h – timed AI) will increase the proportion of cows with complete luteolysis. At day 35±3 postpartum, 70 lactating Holstein cows from one herd were scored for body condition and pre-synchronized with PGF2α and GnRH (3 days apart) and 7 days later submitted to an Ovsynch-56 protocol for first AI after random assignment to two treatments: (1) OV-7 (n=35) with an injection of PGF2α either on day 7; or (2) OV-8 (n=35) on day 8 after G1, respectively. Blood was collected before the first PGF2α, at day 7 and day 8 in OV-7 and OV-8, respectively, at AI and at 7 days after AI to assess progesterone concentration. Ten cows were classified as acyclic and were excluded from the analysis resulting in 60 cows (OV-8, n=27; OV-7, n=33). In total, more (P=0.01) OV-8 cows and more (P=0.04) primiparous OV-8 cows had complete luteolysis compared with their OV-7 herd mates. In addition, more (P=0.008) OV-8 cows with BCS<2.75 had complete luteolysis compared with their OV-7 herd mates, whereas no difference was observed between treatments among cows with BCS ≥2.75. In conclusion, delaying the application of PGF2α by 1 day reduced the percentage of primiparous cows and cows with poorer BCS having incomplete luteal regression at the time of AI.
Currently, many dairy operators have adopted a reproductive strategy known as the Ovsynch protocol that facilitates 100% artificial insemination (AI) submission risk with a predetermined fixed-time AI without the necessity of estrus detection (
1). The Ovsynch protocol consists of an injection of GnRH (G1) at a random stage of the estrous cycle followed 7 days later with a prostaglandin F2α (PGF
2α ) injection and a final GnRH (G2) injection 56 h after the PGF
2α. For maximal pregnancy per AI, timed AI (TAI) should be performed approximately 16 h after G2 administration (
2). Nevertheless, multiple studies have reported that the maximal P/AI that can be achieved with the Ovsynch protocol is limited by at least 3 factors: (a) failure of G1 to induce ovulation of the dominant follicle to initiate a new follicular wave; (b) accomplishment of complete luteolysis after single PGF2α treatment and (c) inability to induce ovulation after G2 (
2). Induction of ovulation in response to G1 at random stages of the estrous cycle occurs in only 50 to 66% of cows (
3). Increased ovulatory response to GnRH is achieved when the Ovsynch protocol was initiated between days 5 and 12 of the estrous cycle (
4). In order to synchronize cows to meet this ideal stage, presynchronization protocols (Presynch, PG-3-G, Double Ovsynch, and GGPG) have been developed (
5,
6). Cows with estrous cycles that were pre-synchronized and that had ovulated after G1 generally have greater P/AI than cows that did not ovulate (
7). Although it was assumed that the latter responses might be a result of an increased circulating P4 concentration from the newly formed corpus luteum (CL) during the development of the preovulatory follicle (
8), the P4 concentration at PGF2α did not differ between cows with one single older CL (one) versus cows bearing an older and an induced CL (two CLs) (
9). Nevertheless, cows that ovulated after G1 and formed a new CL are more likely to have an incomplete luteolysis than cows that did not ovulate because a CL less than 7 days old might still be resistant to regression after a single standard dose of PGF
2α (
10). Indeed, incomplete luteolysis after a single dose of PGF2α has been observed in 5 to 30% of cows submitted either to an Ovsynch protocol (
5,
11) or to a single PGF
2α estrus-synchronization protocol (25%) (
12). Small elevations in circulating P4 near AI (>0.4 ng/mL) resulting from incomplete or delayed luteolysis, have been shown to reduce fertility (
8). Failure of complete CL regression or luteolysis is a greater limitation to achieving maximal P/AI than ovulation failure after G1. Indeed, cows that had a complete luteolysis and ovulated after the G2 have the greatest fertility independent of whether ovulation from the G1 injection occurred (
13).
Therefore, attempts have been made to overcome the problem of incomplete CL regression, by either increasing the dose of PGF
2α (
14) or the frequency of PGF
2α treatments (
15). Increasing the dose of PGF2α (cloprostenol) from 500 to 750 μg for cows submitted to a 7-day Ovsynch protocol increased the luteolytic risk, but this effect was only observed in multiparous cows (
13). Inclusion of a second PGF
2α treatment 24 h after the first PGF
2α (days 7 and 8 after G1), increased the percentage of cows with complete luteolysis from 80% to 97% (16, 15). Although the aforementioned treatments (either increasing the dose or frequency of PGF
2α treatments) have enhanced luteolysis and reduced P4 concentrations at TAI compared with a single standard dose of PGF2α, both increase the cost of the program. In addition, from a practical standpoint, inclusion of a second PGF
2α treatment is time consuming and increases the number of times the cows must be handled during the protocol and thus the risk for compliance.
We hypothesized that a single standard dose of PGF2α injected on day 8 instead of day 7 after G1 will increase the proportion of cows with complete luteolysis and increase the synchronization risk compared with control cows receiving a standard 7-day Ovsynch protocol. Therefore, our objective was to evaluate the effect of a single standard dose of PGF2α treatment on day 8 on the luteolytic and synchronization rates in lactating Holstein cows.
MATERIAL AND METHODS
AnimalsLactating Holstein cows (n=70) in one commercial herd were enrolled in the study. Cows were housed in free-stall barns, fed a total mixed ration once a day to meet or exceed requirements for lactating cows producing 30 L of milk per day. Cows were milked twice daily and had free access to water.
Experimental designStarting at day 35±3 postpartum, all cows were scored for body condition (BCS) on a five-point scale (1 = emaciated and 5 = obese) (
17). Estrous cycles were pre-synchronized using PG-3-G protocol (
9). The protocol consisted of a 25 mg i.m. injection of PGF
2α (Pre-PG; 5 mL of Dinolytic, Zoetis Animal Health, Madison, NJ) followed by an i.m. injection of GnRH (8 μg Buserelin – a GnRH agonist, Pre-GnRH; 2 mL of Receptal, MSD Animal Health, Intervet, International, GMBH, Germany) 3 days later. Ten days after the Pre-PG injection, an Ovsynch TAI program was initiated and cows were allocated randomly to two treatments: OV-7 (n=35, G1–7 days – PGF2α – 56 h – G2 – 16 h – TAI)) with an injection of PGF
2α on day 7 and OV-8 (n=35, G1–8 days – PGF2α – 56 h – G2 – 16 h – TAI) with an injection of PGF
2α on day 8 after the G1, respectively. Transrectal ultrasonography was conducted at G1 to map ovarian structures, at PGF
2α to assess ovulation in response to G1, and at 7 days after TAI to assess ovulation in response to G2.
Blood samples to assess concentrations of P4 were collected at Pre-PG, G1, PGF
2α, at 72 h after PGF
2α (at TAI) and at 7 days after TAI from the coccygeal vein or artery into evacuated tubes (BD Vacutainer® Plymouth, UK). The samples were refrigerated immediately and transported in a portable refrigerator to the lab by 3 h after collection. Tubes were centrifuged (1,000 x g for 15 min), and serum was separated and stored at -20 °C until P4 analysis. The P4 analysis was done at the Faculty of Veterinary Medicine – Skopje, North Macedonia using a commercially available kit (HUMAN, Progesterone ELISA Test – GMBH, Germany) on an Immuno-scan BDLS reader. The intra- and inter-assay CV were 7.3% and 8.4%, respectively.
Cyclic status of the cows (cyclic or acyclic) was determined by serum concentrations of P4 collected at Pre-PG and at G1. When concentrations of both samples were <1 ng/mL, the cows were classified as acyclic and data were not included for further analyses (8 OV-8 cow and 2 OV-7 cows) (
9). In contrast, when either of the paired samples at Pre-PG and G1 contained concentrations of progesterone ≥1 ng/mL, cows were classified as cyclic (
9). In cyclic cows in which progesterone concentration was ≥1 ng/mL at PGF2α or at 7 days after TAI, it was furthermore assumed that ovulation was induced by injections of G1 or G2. Complete luteolysis was defined to occur when P4 was >1 ng/mL at PGF
2α and <0.4 ng/mL 72 h later at TAI (
9). Synchronization risk (SR) was defined as the number of cows with complete luteolysis and ovulation success after G2, divided by the number of cows with progesterone >1 ng/mL at PGF
2α (
9).
Statistical analysesAll statistical analyses were performed with SPSS (version 22.0, IBM SPSS Statistics for Windows, Armonk, NY). Continuous variables such as progesterone concentrations were analyzed using GLM Univariate procedure in SPSS. Because these data were not normally distributed, a twostep transformation to normality was conducted before the analysis. Firstly, a fractional ranking of the data was performed followed by application of the inverse distribution function (IDF. NORMAL) of SPSS.
Variables with a binomial distribution (complete luteolysis, SR, and BCS [<2.75 or ≥2.75]) were analyzed by binary logistic regression. For these variables, data were analyzed for all combinations of parities and treatments. The same method was also used to analyze the relationship between circulating P4 concentrations and the binomial traits. The relationship between the P4 concentrations, treatment, and BCS, was assessed by two multiple linear regression analyses using the GLM Univariate procedure in SPSS. Two models were constructed which included P4 concentration at G1 and at PGF
2α as independent variables in each model separately, treatment as fixed effect, and BCS as covariate in both models. Statistical differences were considered significant at P≤0.05 and as a tendency for P≤0.10.
RESULTS
Of 70 cows, 10 cows (14.2% [n=8] in OV-8 and OV-7 [n=2]) in both treatments were classified as acyclic and were therefore excluded from further analyses leaving a total of 60 cows (OV-8, n=27, OV-7, n=33).
The concentrations of P4 at PGF2α treatment did not differ (P=0.62) between the OV-7 and OV-8 (6.1±0.57 and 6.6±0.59 ng/mL, mean±SEM), respectively. Similarly, when P4 concentrations at PGF2α were compared between parity groups within treatment, no differences were detected between primiparous (P=0.82) nor between multiparous cows (P=0.65).
When the P4 concentration at G1 was compared between OV-8 and OV-7 (5.1±0.41 and 4.6±0.39 ng/mL), respectively, no difference (P=0.32) was detected. In addition, the P4 concentrations at G1 did not differ between parity groups within treatments for primiparous (P=0.90) or multiparous cows (P=0.25).
Nevertheless, a significant difference was observed in the percentage of cows with complete luteolysis between treatments, with more cows having a complete luteolysis in OV-8 compared with OV-7 (
Table 1). In addition, a tendency was observed for increased CL regression in multiparous cows in the OV-8 versus the OV-7 treatment. Within the OV-7 group, multiparous cows tended (P=0.09) to have a greater proportion of animals with complete luteolysis. When only primiparous cows from both treatments were analyzed, a greater (P=0.04) percentage of OV-8 cows had complete luteal regression compared with OV-7 cows, whereas no differences were observed between parities among the OV-8 cows (
Table 1).

No differences in SR were detected between treatments or parity groups (
Table 2).
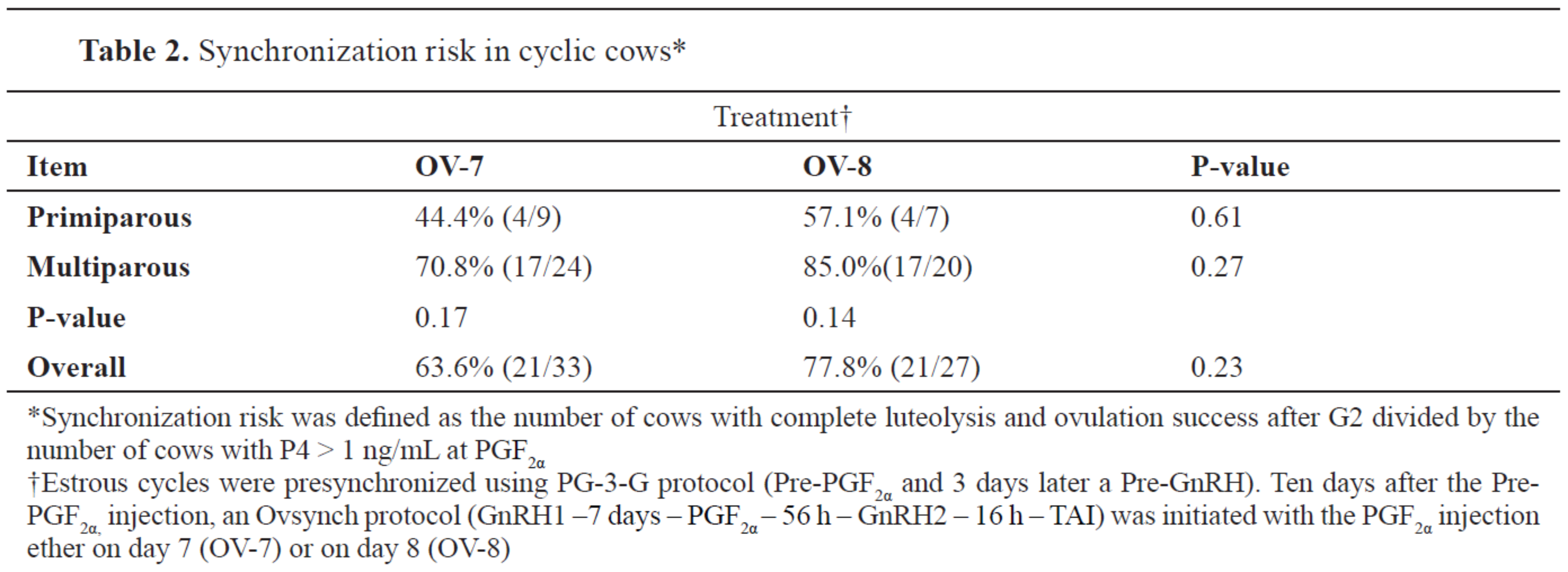
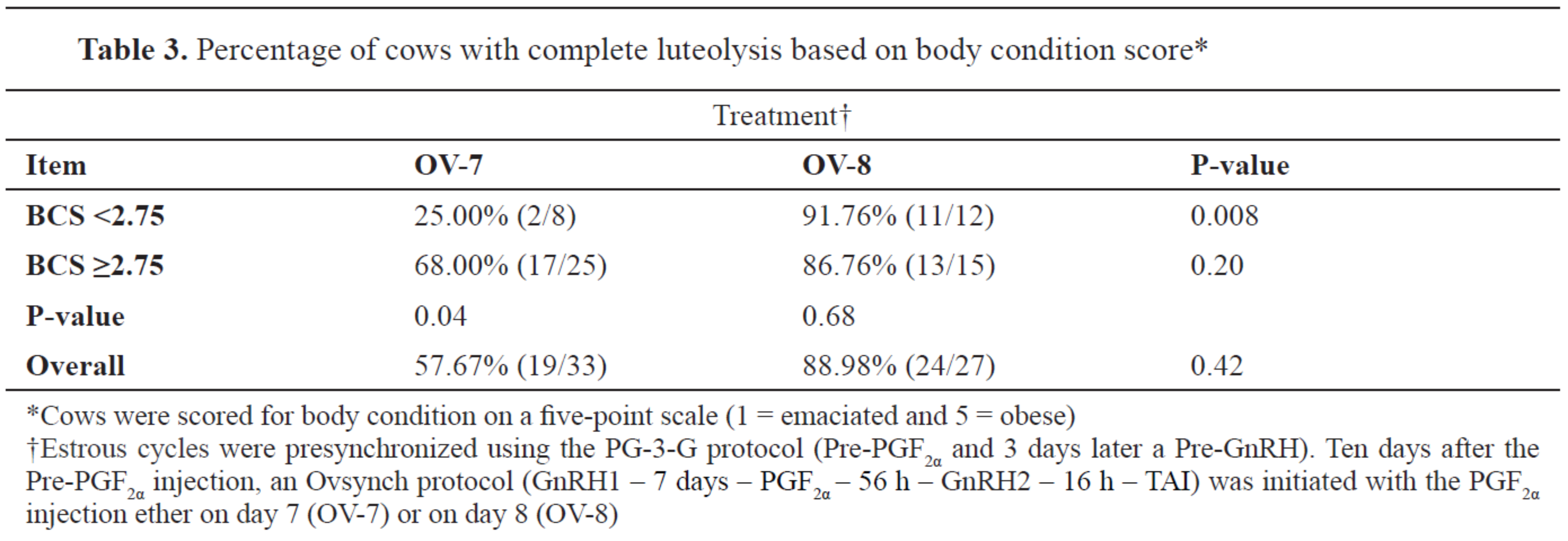
When the cows from both treatments were categorized based on their BCS (<2.75 and ≥2.75), overall no differences in complete luteolysis were observed (
Table 3). Cows in OV-8 having BCS <2.75 were more likely to have a complete luteal regression compared with their OV-7 herd mates, whereas no difference in complete luteolysis was observed between treatments when BCS ≥2.75. In the OV-7 treatment, cows with BCS ≥2.75 were more likely to have a complete luteolysis compared with their leaner group mates.
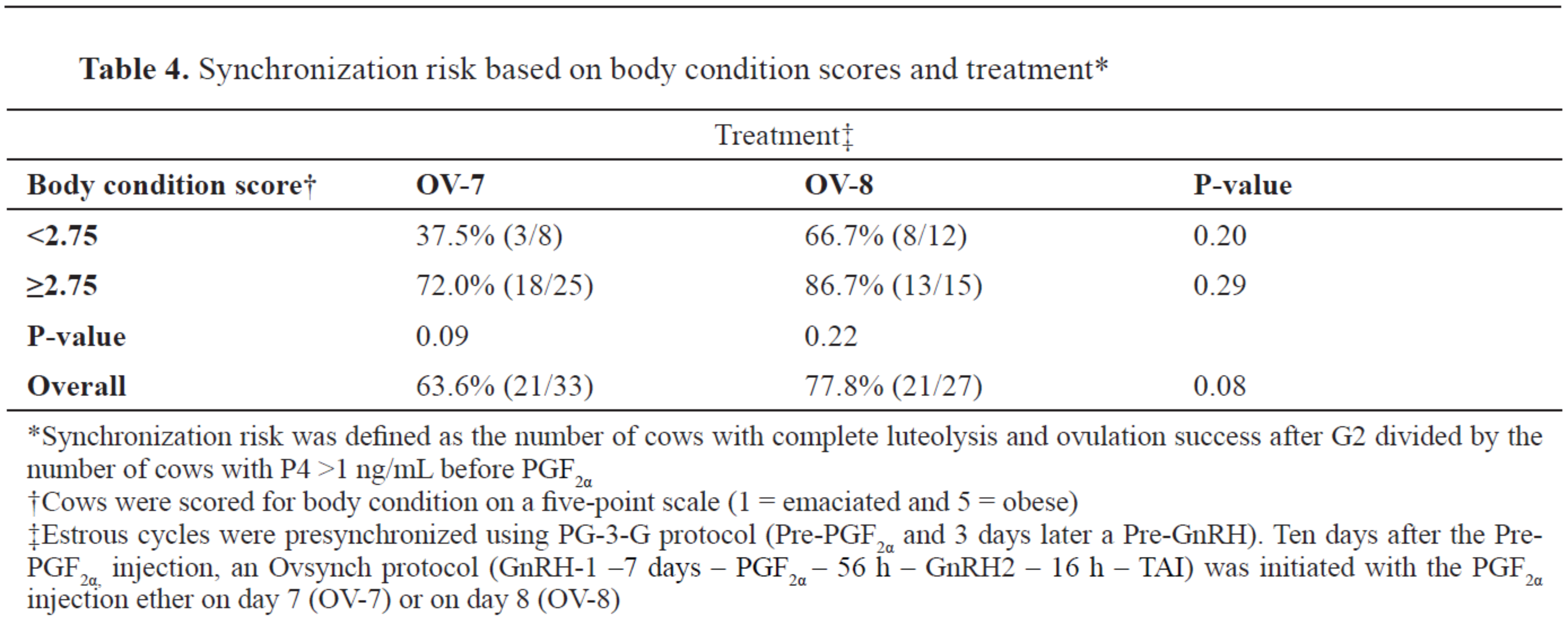
In addition, a tendency (P=0.08) was observed for increased SR in OV-8 cows compared with OV-7 cows (
Table 4). In addition, in the OV-7 treatment group, there was a tendency (P=0.09) for a greater percentage of synchronized cows in the BCS ≥2.75 group in comparison to their group mates with a lower BCS (
Table 4).
Progesterone concentrations also were associated with the BCS at G1 and at PGF
2α. At G1, for each unit increase in BCS (in the range of 2.00 to 3.75), serum concentration of P4 increased (P=0.03) by 1.68±0.77 ng/mL. At PGF
2α for each unit increase in BCS (in the range 2.00 to 3.75), serum concentrations of P4 increased (P=0.01) by 3.1±1.16 ng/mL.
DISCUSSION
The present study aimed to determine the efficacy of injecting PGF
2α on day 8 instead of day 7 to reach a greater percentage of complete luteal regression in the standard Ovsynch protocol in lactating dairy cows. Overall, a greater percentage of OV-8 treated cows had complete luteal regression compared with OV-7 cows. The percentage of OV-8 cows having complete luteal regression observed in our study was similar to that observed in other studies (
16,
18); in which 80 to 90% of the enrolled cows had complete luteal regression using a 7-day protocol. The latter implies that PGF
2α injection on day 8 could be more effective than injection on day 7 in the standard Ovsynch protocol to induce complete luteal regression.
Our results indicate a 34.3 percentage unit increase in luteal regression (88.9% vs. 57.6%,
Table 1) after the OV-8 treatment compared with OV-7 treatment, respectively. Increased luteal regression in response to the OV-8 treatment could be attributed to the presence of an original CL in combination with a newly formed CL in response to G1 and the maturity of both CL. One study (
14) reported that the presence of an older and a newly formed CL has a major effect on whether cows will have complete luteal regression. In that study, only 79.4% of the cows having a new G1-induced CL had a complete CL regression, compared with 93% complete CL regression in cows without a new CL. In contrast, Stevenson et al. (
9) reported that the newly formed CL regresses more successfully in the presence of a more mature CL. Regardless of the pathways involved, regression of all luteal structures primarily depends on their maturity at the time of the PGF
2α injection (
10). Because we did not include the data regarding the presence of one or more CL on the ovaries at the time of PGF
2α treatment, we are not able to confirm these observations. Nevertheless, in the present study, when ovulation occurred in response to G1 in the presence of an original CL, the newly formed CL and the older CL would be approximately 7 and 14 days old, respectively at PGF
2α in the OV-8 treatment. In this scenario, both CL should be fully responsive to PGF
2α resulting in an increased likelihood for complete luteal regression. In the same scenario for the OV-7 treatment, the older and newly formed CL would be approximately 6 and 13 days old respectively, at PGF
2α (
19). Because CL less than 7 days are more resistant to regression after PGF
2α (
10), fewer cows with complete regression would be expected.
When parity was included in the analysis, the results indicated a greater proportion of multiparous cows had complete luteal regression compared with primiparous cows in the OV-7 treatment (
Table 1). In contrast, in one report, a greater proportion of primiparous cows had a complete luteolysis in response to a standard dose of PGF
2a compared with multiparous cows (94 vs 81%, respectively) (
18), whereas other studies reported no effects of parity (
9,
15). Although, the reasons are still unclear, we hypothesize that some follicles that eventually formed a new CL during a PG-3-G + OV-7 protocol in primiparous cows may be smaller, resulting in a smaller and less functionally mature CL (
20,
21) and therefore represent a CL with a lower probability of complete regression.
Particularly intriguing in our analysis was that a greater proportion of primiparous cows (66.7%) in OV-7 treatment that did not have complete luteal regression compared with only 14.3% of the OV-8 cows. We were unable to find any other studies reporting similar results, thus these intriguing findings warrant further investigation. Nevertheless, as mentioned previously, ovulation of small follicles resulting in a less mature CL could be a possible explanation that contributes to inadequate luteolysis.
Although complete luteolysis was less successful in the OV-7 cows, the greatest failure was among thinner cows (BCS<2.75). These results do not corroborate the results reported by Stevenson and Phatak (
22), in which thinner cows were more likely to have complete luteal regression than cows with greater BCS. It is unclear how body condition might influence luteolysis; however, it is possible that a less functional follicle ovulated in thinner cows and formed a CL that was 6-days old at the time of PGF
2α treatment and less sensitive to the luteolytic effects of PGF
2α. The SR tended to be greater for OV-8 than OV-7 cows. We found no report in the literature corroborating the effect of synchronization risk on luteolysis. Nevertheless, from a practical point of view, these findings should be taken into consideration, when thinner cows are submitted to the standard Ovsynch protocol.
CONCLUSION
Treatment with PGF
2α on day 8 rather than day 7 during the PG-3-G Ovsynch protocol reduced the percentage of cows with incomplete CL regression at the time of AI and tended to increase synchronization risk. The treatment was also successful in primiparous cows and in cows with lesser BCS. Based on our results, we suggest that this treatment could be of practical importance for many dairy farms. Further studies are warranted, however, to confirm these demonstrated improvements in CL regression and evaluate potentially concomitant increases in pregnancy per AI in response to this novel OV-8 treatment.
CONFLICTS OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTS
We would like to acknowledge ZK Pelagonija Dairy Farm (Bitola, R. M) for allowing us the use of their facilities and Zoetis Animal Health Inc. (Madison, NJ) for donating Dinolytic used in this research.
AUTHORS’ CONTRIBUTION
BA, TD, JS and GO planed the study design. BA and TD performed the ultrasonographic examination of the cows. GO and JS performed the proofreading. JS and NA have run the statistical analysis and IC has run the laboratory analysis. KI and BA have synchronized the cows. ZT and FD collected the blood samples.

 10.2478/macvetrev-2020-0028
10.2478/macvetrev-2020-0028



