Studies on caprine leptospirosis using isolation, histochemistry and immunohistochemistry are rare. The role of small ruminants in the epidemiology of leptospirosis is scarce. This study investigated the prevalence of Leptospira spp. serovars, and the renal pathology of caprine leptospirosis in slaughterhouses from two states in southwest Nigeria using isolation (IS), Warthin Starry silver (WSs) impregnation and immunohistochemistry (IH). One hundred and sixty-nine kidney samples were randomly obtained from goats between September 2015 and June 2017. Chi-square test was used with a confidence level set at 0.05 to ascertain associations between the positive cases, sex and animal species. Eightyseven (51.5%) samples were positive on IS, out of which 26/40 and 25/30 were positive on WSs and IH, respectively. Ten (5.9%) kidneys showed macroscopic lesions while interstitial nephritis (48.6%) and tubular nephrosis (64.2%) were the most prominent histopathological changes. The most frequently observed positive reactions were against serovars Hardjo type Prajitno (12/25, 48%), and Gripptotyphosa (5/25, 20%). Other serovars such as Bratislava (2/25, 8%), Canicola (3/25, 12%), Icterohaemorrhagiae (2/25, 8%), and Pomona (1/25, 4.0%) were also detected using IH. The result showed high prevalence of Leptospira infection in goats and the possibility of humans contracting the disease. To date, the detection of leptospirosis from kidneys of goats using IS, WSs and IH has not been reported. This study is the first documentation of evidence of pathogenic Leptospira species in renal tissues of goats.
The importance of goats in the sub-Sahara livestock industry cannot be underestimated (
1). It represents about 48.9% of the total grazing livestock in Nigeria (
2). In Nigerian society, goats are raised for their meat, milk, hair and skin. They also serve as a flexible financial reserve for the rural population as well as for the socio-cultural customs and traditions of the people (
1). In tropical regions, goat farming is a long and common household practice where animals feed on kitchen wastes, scavenging, and bush grazing resulting in low productivity and high rate of disease incidence (
3).
In southwest Nigeria, goat production systems are free-roaming, tethering and confined. Goats are bred by a large number of rural households, more frequently as free-roaming village flocks and more rarely in pens. Usually, individuals have two to four animals of which two or three are shegoats, depending on the number of available bucks for reproductive activities (
4). Over the years, in the humid region of sub-Saharan Africa, diseases have been the major constraint in small ruminant production, especially peste des petits ruminants (PPR) (
5). Regrettably, the impact of other diseases like leptospirosis has not been fully investigated.
Leptospirosis has been adjudged to be one of the most widespread diseases worldwide (
6). But the impact of the disease is uncertain especially in many developing countries of the world. The prevalence of animal infection across the world, estimated by serological analysis, is between 2% to 46%, depending on the animal species (
7). The disease is of major economic concern when it involves a reproductive failure in food-producing animals (
8). In Brazil, it was described as the most frequent infection with a high potential of impairing productivity in small ruminants (
9). Leptospirosis mimics other infectious diseases including brucellosis, trypanosomosis, influenza, Q-fever, ehrlichiosis and anaplasmosis (
10). This has led to misdiagnosis and under-reporting of the disease in a flock or herd of small ruminants (
11,
12). The epidemiology of leptospirosis in goats is uncertain, but it is possible that goats could serve as a source of infection to humans since they live in close proximity with their owners (
13). The disease usually produces subtle and subclinical infections in goat herds. Acute infection is rarely observed in adult goats but the subclinical infection is mainly characterized by clinical manifestations such as decreased milk production, abortions, low fertility, weak kids (
14,
15), as well as non-reproductive death (
16). In the pathogenesis of leptospirosis, the kidney has always been one of the ‘preferred’ target organs in both acute and chronic infections. The acute injury is as a result of tubulointerstitial nephritis (
17). A previous study in southwest Nigeria on leptospirosis in sheep and goats more than seventeen years ago indicated a seroprevalence of 13.1% (
13). There is a paucity of information on the prevalence of caprine leptospirosis and the associated serovars, as well as the renal pathology in Nigeria due to the insufficient employment of improved diagnostic methods. Studies on caprine leptospirosis using culture isolation, histochemistry and immunohistochemistry are rare. Therefore, this study was designed to determine the prevalence of caprine leptospirosis, associated serovars, and the renal pathology in slaughterhouses from two states in the southwest Nigeria employing culture-isolation (IS), Warthin Starry silver impregnation (WSs) and immunohistochemistry (IH).
MATERIAL AND METHODS
Study locationThe study was carried out in southwest Nigeria. The area consists of three local government areas within two neighboring states (Oyo and Ogun) characterized by a humid tropical climate with two distinct seasons; a long rainy season (March to November) and a short dry season (December to February). This study was carried out within the cities of Ibadan and Abeokuta, the capitals of Oyo and Ogun States, respectively (
Fig. 1).
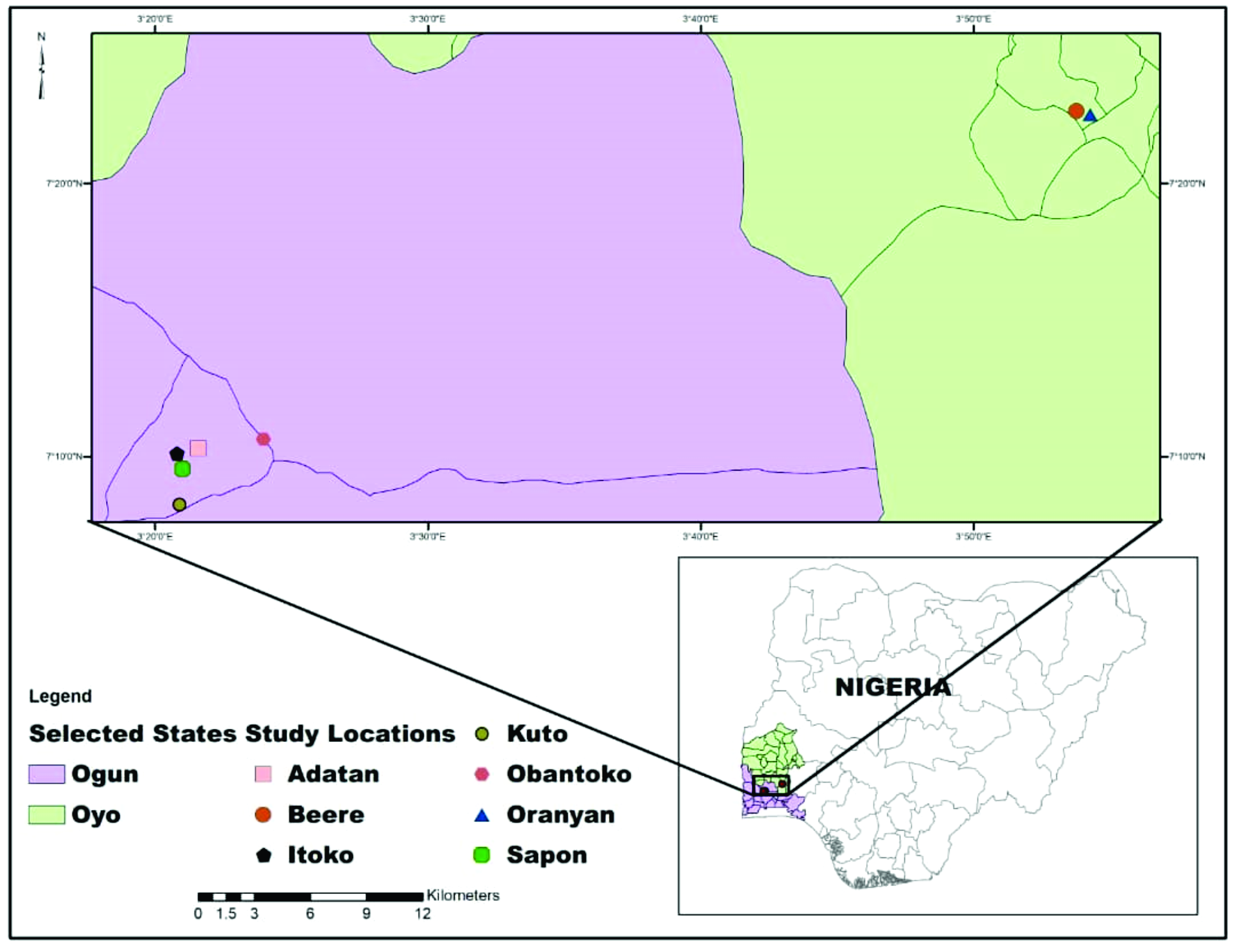 Figure 1.
Figure 1. Map of the study area showing locations of sample collection
The city of Ibadan is located on the latitude of 7°23’47’’N, and longitude of 3°55’0’’E with a mean total rainfall of 1420.06 mm3, mean temperature of 23.94 °C and relative humidity of 74.55%, with a population of over 4 million people. The city of Abeokuta is located on the latitude of 7°9’39’’N and longitude of 3°20’54’’E, with a mean annual rainfall of 1037.00 mm3, a relative humidity of 82% and mean of 30 °C. The population of Abeokuta and its environs is approximately 1 million people.
Data sourceThis study was conducted on goats raised and managed by indigenous people of Beere and Oranyan in Ibadan southeast local government of Oyo state and the surrounding towns and villages, while those from Ogun-state were from Obantoko, Adatan, Kuto, Sapon and Itoko in Odeda, and Abeokuta south local government areas (
Fig. 1). West Africa dwarf (WAD) goats are kept by a large number of rural households as free-roaming village flocks, while the Red Sokoto were from the Northern parts of the country. The ownership pattern for the WAD is usually 2-4 animals per individual (
4). The kidney samples were obtained from various slaughter slabs within the cities of Ibadan and Abeokuta.
Sample size determinationThe sample size was calculated with an estimation of the predicted prevalence of 13% having an accuracy of 5% at a 95% confidence interval. The calculations were made as described by Cannon and Roe (
18).
N = 1.96² P
exp (1- P
exp)/d²
Where N = sample size required
P
exp = predicted prevalence
D = desired precision (5%)
1.96 = constant for stratified and simple random samplings
Sample collectionA total of 169 kidney samples were randomly obtained from goats slaughtered in these slaughter houses. One hundred and thirteen (n=113) kidney samples were obtained from the slaughter slabs at Oranyan in Ibadan, and 56 kidney samples were obtained from Obantoko, Adatan, Kuto, Sapon and Itoko slaughter slabs in Abeokuta, Ogun State. The samples were collected between September 2015 and June 2017. Parameters such as breed, sex and age were recorded. The approximate age of the animals was determined by the dentition. The samples were placed in sterile polythene bags and put into ice packs and transported to the Department of Veterinary Pathology, Federal University of Agriculture, Abeokuta within 3 to 4 hours of collection for subsequent bacteriological and pathological analyses.
Culture isolation and culture mediumEllinghausen-McCullough-Johnson-Harris broth medium (EMJH) (Difco®-USA) with the addition of 10% filtered rabbit’s serum (0.2 μM sterile filter), magnesium chloride (1 ml) and calcium chloride (1 ml) was used for isolation of the organisms (19, 20). Nalidixic acid (50 mg/L; Inlab®-BR), neomycin (10 mg/L; Sigma®-USA) chloramphenicol (5 mg/L; Sigma®-USA) and 5-fluorouracil (400 mg/L; Sigma®-USA) was added to prevent the growth of contaminating bacteria.
Leptospira isolationThe isolation of
Leptospira was performed by maceration of about 0.2-0.3 g of kidney in 2-3 ml of Phosphate Buffer Solution (PBS) with toothed forceps. This was allowed to stay for 10-15 minutes to allow the
Leptospira organism to move out of the tubules. Direct inoculation of four to five drops of the macerate into 5 ml of Ellinghausen- McCullough–Johnson–Harris broth medium (EMJH) (Difco®-USA) was performed. The inoculated EMJH medium was incubated at room temperature (28-30 °C) in the dark and examined under darkfield illumination daily and weekly for the growth of the organism for three months (
21).
Pathological evaluationsThe 169 kidneys were macroscopically examined and processed for histopathological examination. Paraffinized tissues were routinely stained with Hematoxylin and Eosin. Forty (n=40) kidneys out of the eighty-seven (n=87) culture-positive samples for
Leptospira in the EMJH medium were selected for Warthin Starry silver (WSs) impregnation technique (
22). The presence or absence of histopathological changes was scored as positive (+) or negative (-), respectively. The severity of the histological lesions such as tubular degeneration and necrosis (TDN), interstitial nephritis (IN), tubular dilatation (TD) and hyaline cast (HC) were graded and scored as follows: 1 = mild (a focal area on section examined), 2 = moderate (3–4 foci on section examined), 3 = severe (> 5 foci on the lesion examined).
ImmunohistochemistryOut of 40 kidney samples used for WSs technique, 30 were subjected to IH to determine the serovar and exact location of
Leptospira antigen within the renal parenchyma. The six serovars of rabbit monoclonal antibodies used for immunohistochemical detection were Hardjo type Prajitno, Canicola, Grippotyphosa, Bratislava, Pomona and Icterohaemorrhagiae (
Table 1). The rabbit immune sera-cocktail (
Table 1) used as primary monoclonal antibodies against
Leptospira antigens in the kidney sections were acquired (WHO/FAO/OIE and National Leptospirosis Reference Centre, KIT Biomedical Research, Amsterdam, The Netherlands). Serial 5 μM sections from each paraffin block were mounted on slides and allowed to dry overnight. The sections were deparaffinized in 3 changes of xylene for 3 min each, rehydrated in a graded series of ethanol solutions (100%, 95%, 70%, 50% v/v), and finally washed with deionized water. The deparaffinized sections were then placed in a Coplin-jar containing 0.01 M sodium citrate buffer (pH 6.0) and heated for 40-min periods, followed by cooling to room temperature for 20 minutes, to unmask the
Leptospira antigens. Following 5 minutes of washing in 0.05 M Phosphate-buffered saline solution (pH 7.6) containing 0.05% Tween 20 (TPBS). All steps were performed at room temperature. The sections were first incubated in 3% hydrogen peroxide for 15 min to suppress endogenous peroxidase. After a brief wash in TPBS, nonspecific binding was blocked by bathing in normal goat serum for 10 min.
The tissues were then incubated with rabbit
Leptospira monoclonal (1:800 dilution in PBS) antibodies (serovars Pomona, Bratislava, Icterohaemorrhagiae, Hardjo, Canicola, Gripptotyphosa) overnight. After a brief wash in TPBS, the slides were incubated with biotinylated secondary antibody (goat anti-rabbit IgG) for 15 min. After the last wash in TPBS, the slides were incubated with streptavidin–biotin–horseradish peroxidase for 15 min. Slides were then rinsed with distilled water and incubated with chromogen for 10 min. Slides were finally rinsed with distilled water to stop the staining process. Sections were counterstained with Mayer’s hematoxylin for 3 min followed by a 5-minutes rinse in running tap water and were mounted with glycerol water-soluble mounting medium for microscopic evaluation. Negative controls were slides from
Leptospira-infected tissues that were subjected to the same staining procedure as the others except that normal rabbit serum was used in place of the monoclonal antibody.
Statistical analysisData were presented using descriptive statistics. Effects of age, breed, sex and location were determined using Chi square. P value <0.05 was considered significant. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS).
RESULTS
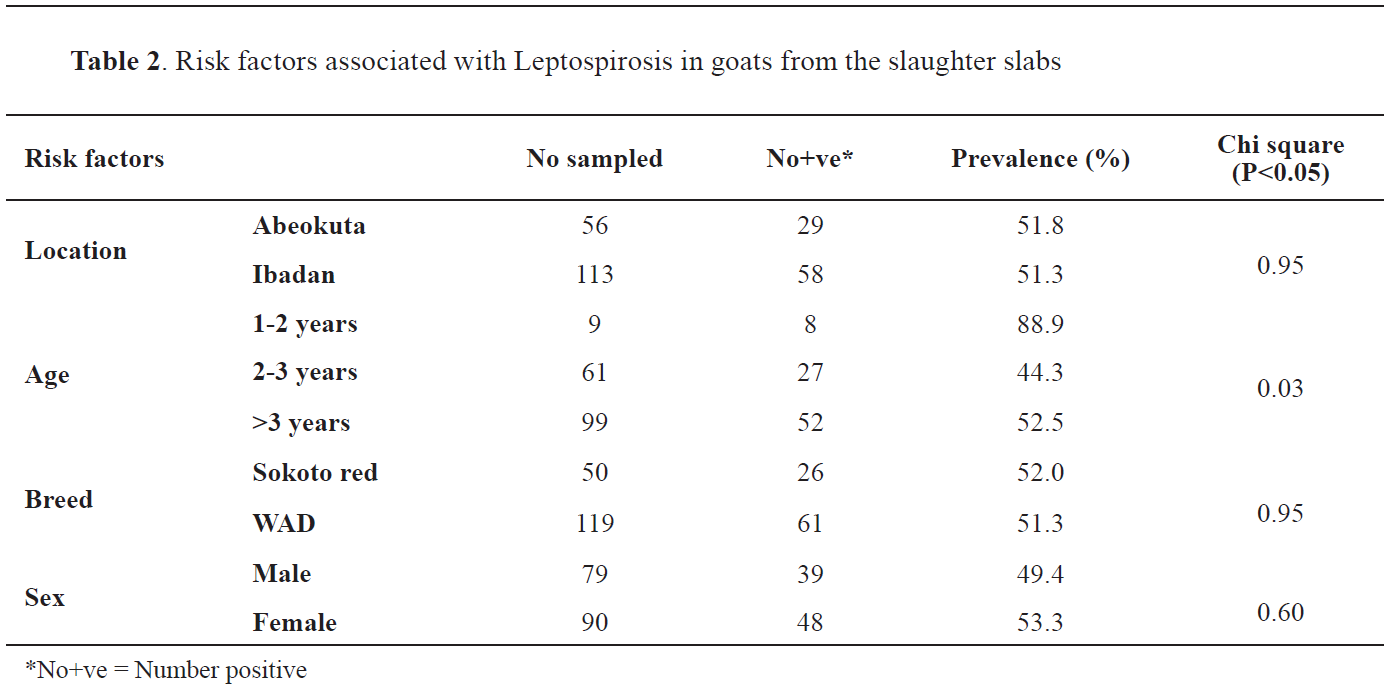
The overall prevalence of caprine leptospirosis from the study area was 51.5% (87/169). Out of the 56 kidney samples obtained from Abeokuta, 29 (51.8%) were positive for
Leptospira infection while 58 (51.3%) were positive out of the 113 kidney samples obtained from Ibadan. There was no significant difference (P>0.05) between location and the prevalence of caprine leptospirosis. Out of the 9 kidney samples obtained from goats between 1-2 years of age, 8 (88.9%) were positive for
Leptospira infection while 27 (44.3%) out of the 61 samples obtained from goats between 2-3 years were positive for leptospirosis. Out of the 99 kidney samples obtained from goats above three years, 52 (52.5%) samples were positive for
Leptospira infection. There was a significant (P<0.05) difference between the animals’ age and the prevalence of leptospirosis. The prevalence of leptospirosis in Red Sokoto breeds was 52.0% out of the 50 samples examined, while 61 (51.3%) out of 119 kidney samples from WAD goats were positive for leptospirosis. There was no significant difference (P>0.05) between goat breed and the prevalence of leptospirosis. Out of the 79 kidney samples from male goats, 39 (49.4%) were positive for leptospirosis, while 48 (53.3%) were positive out of the 90 kidney samples from female goats. There was no significant difference (P>0.05) between sex and the prevalence of
Leptospira infection (
Fig. 2) (
Table 2).
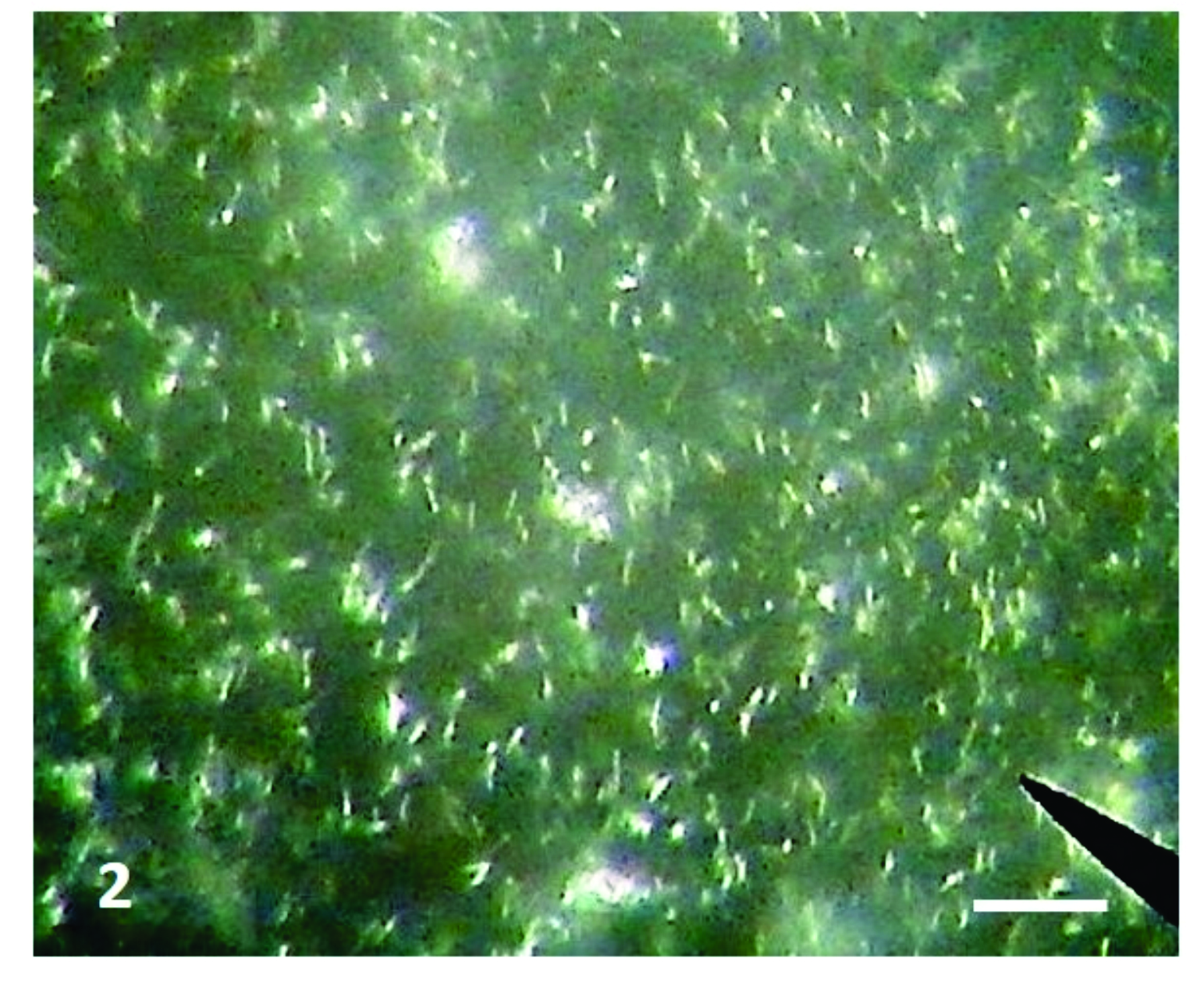 Figure 2.
Figure 2. Photomicrograph of cultured
Leptospira organism. Dark field microscopy; x400, Bar=100μm
 Macroscopic lesions
Macroscopic lesionsOut of the 169 kidney samples obtained, only 10 (5.9%) revealed gross morphological changes. The type, severity and distribution of the changes are depicted in
Table 3. Out of the 10 kidney samples with gross morphological alterations, 4 had focal to multiple foci of mild cortical petechial bleeding, 3 had multiple foci of pale necrotic areas and 3 showed mild to moderate rough pitted cortical surfaces. The remaining 159 kidney samples showed no gross lesions.
Renal histopathologyThe histological lesions observed in the kidneys were interstitial nephritis (characterized by interstitial, peri-glomerular and perivascular lymphoplasmacytic infiltrates) (
Fig. 3a), tubular necrosis, protein casts (
Fig. 3b), hemorrhages, multiple foci of interstitial fibrosis (6.2%), tubular dilatation (21.0%), tubular calcification (7.0%), and tubular atrophy (16.9%). Tubular epithelial necrosis (64.2%) and interstitial nephritis (48.6%) were the most frequent histological lesions observed (Table 3).
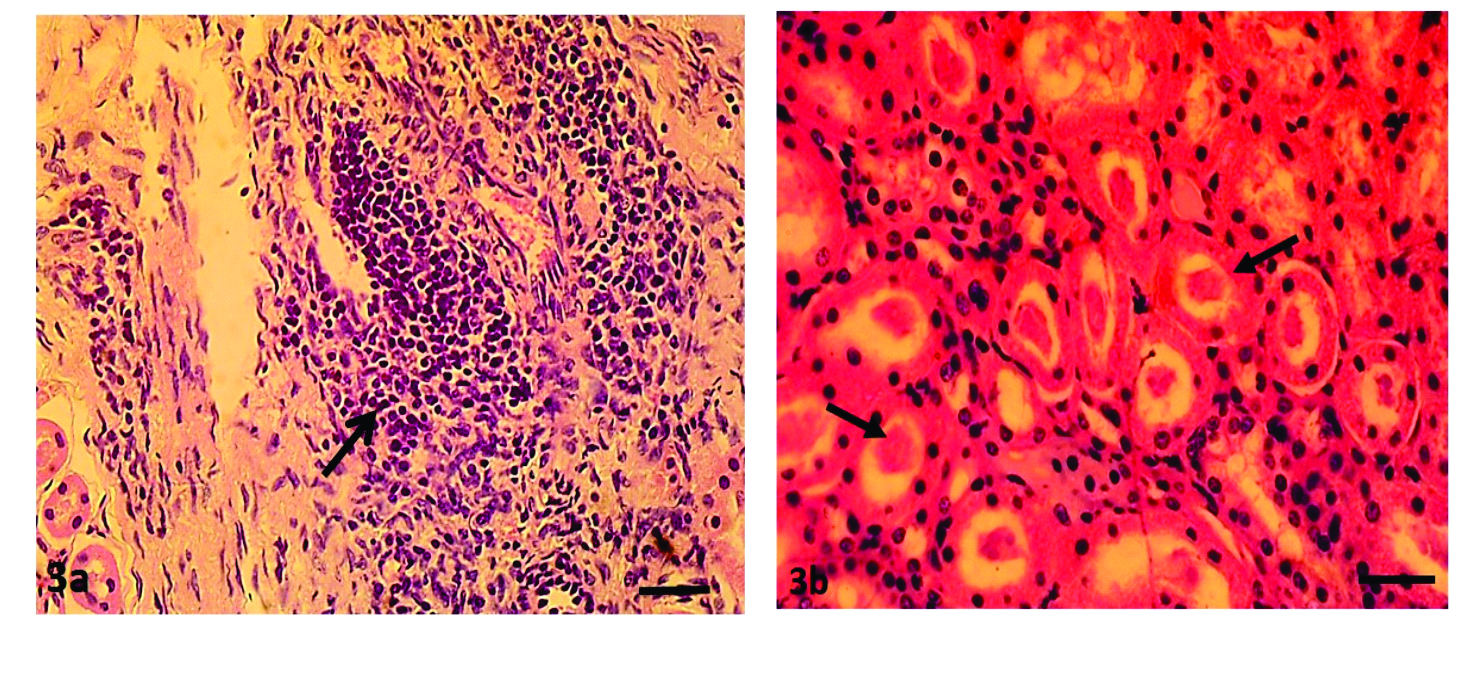 Figures 3a and 3b.
Figures 3a and 3b. Kidney showing severe tubular degeneration and necrosis, tubular atrophy and effacement due to marked focal interstitial and perivascular mononuclear cellular infiltration (a) and showing diffuse tubular degeneration and necrosis with protein casts within the tubular lumina (b). H&E; x400, Bar=120μm
Tubular necrosis, tubular dilatation, and protein casts were mild to marked in severity while the degree of interstitial nephritis was only mild to moderate. Out of the 40 SI-positive kidney sections subjected to WSss, 26 (65%) demonstrated the presence of the organism.
Leptospira were slightly brown to deep brown (
Fig 4a) and in some instances appeared as a black thread (
Fig 4b) or dots within the tubular lumen or closely attached to the apical portion of the tubular epithelial cells.
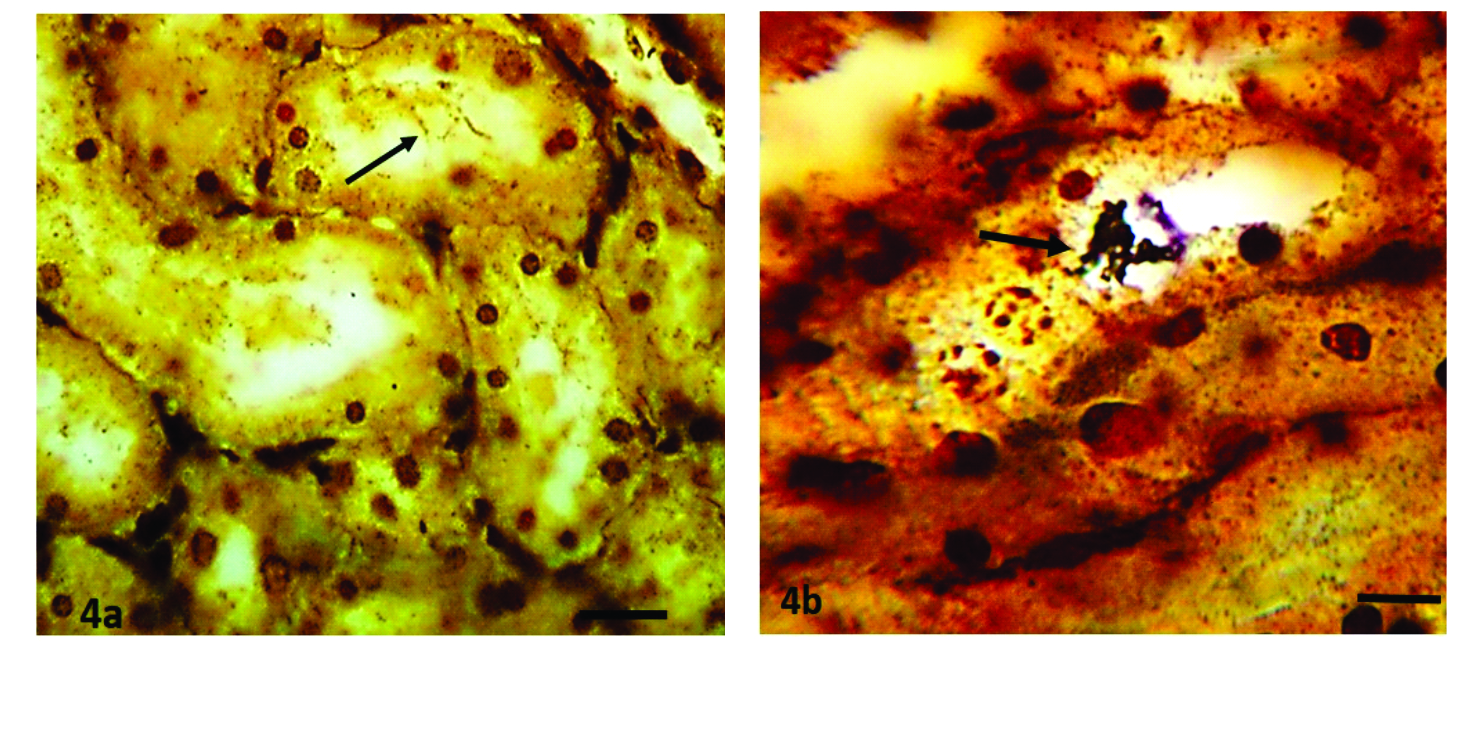 Figures 4a and 4b.
Figures 4a and 4b. Kidney sections showing the presence of
Leptospira organisms within tubular lumina (arrows). Warthin Starry silver; x400, Bar=150μm (a), 75μm (b)
Immunohistochemical evaluationOut of the 30 kidney samples positive on IS for
Leptospira and analyzed on IH, only 25 (83.3%) samples were immunoreactive (
Table 3). Out of the 25 positive samples, 12 (48%) were immunoreactive to serovar Hardjo type Prajitno (
Fig. 5a and b), 5 (20%) for Gripptotyphosa, 3 (12%) for Canicola (
Fig. 5c), 2 (8%) each for serovar Bratislava and Icterohaemorrhagiae, and 1 (4%) for serovar Pomona.
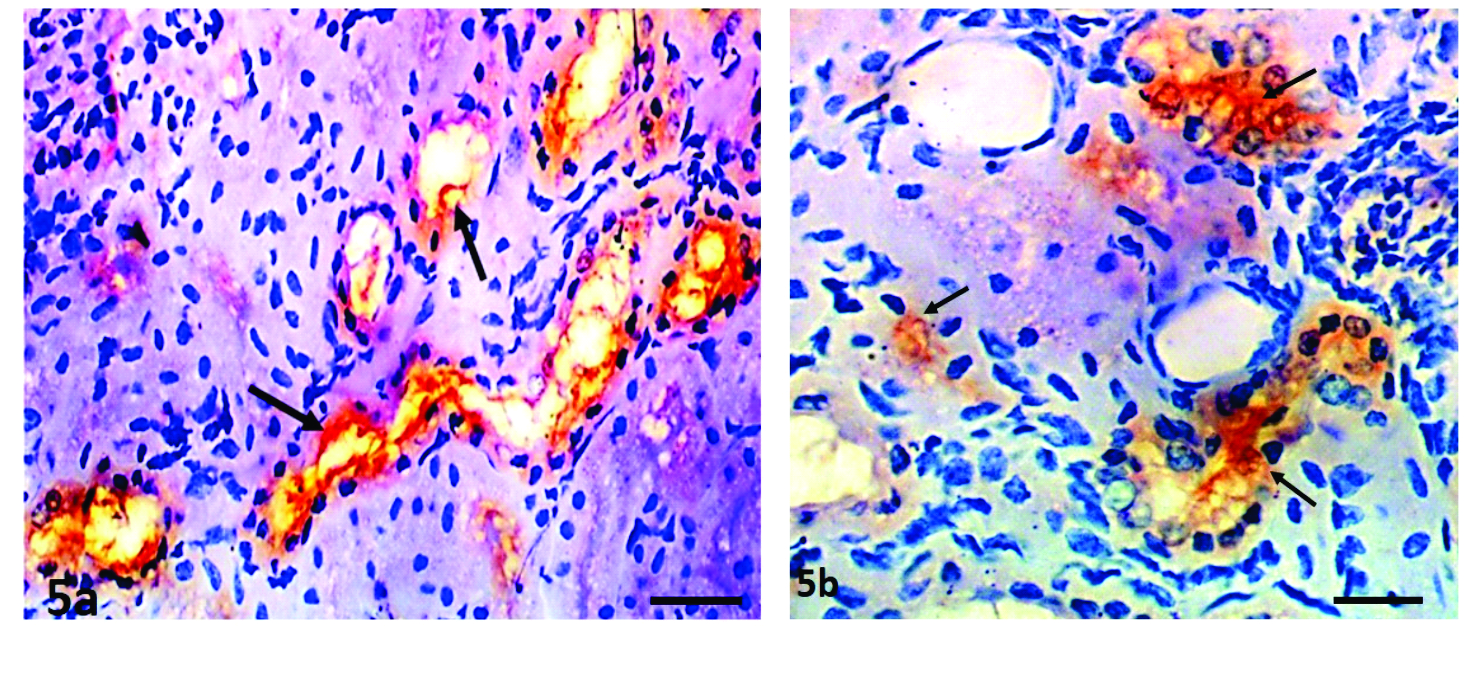 Figures 5a and 5b.
Figures 5a and 5b. Kidney sections showing
Leptospira Hardjo colonization of the collecting tubules (arrows) and the lumina of distal convoluted tubules. Streptavidin-biotin immunoperoxidase; x400, Bar=180μm (a), 120μm (b)
 Figure 5c.
Figure 5c. Kidney sections showing
Leptospira canicola colonization of the collecting tubules (arrows). Streptavidin-biotin immunoperoxidase; x400,
Bar=100μm
The positive immunoreactivity was observed either closely confined within the tubular lumina or on the apical surfaces of the tubular epithelial cells, and was not observed in the adjacent peritubular tissues and interstitium. Wavy and dot-like appearance was observed within the renal tubular lumina. Twenty kidney sections which were positive on IS and WSs also showed foci of immunoreactivity with at least one monoclonal antibody (
Table 3). Five kidney sections were WSs-negative but were positive on IS and IH (
Table 3).
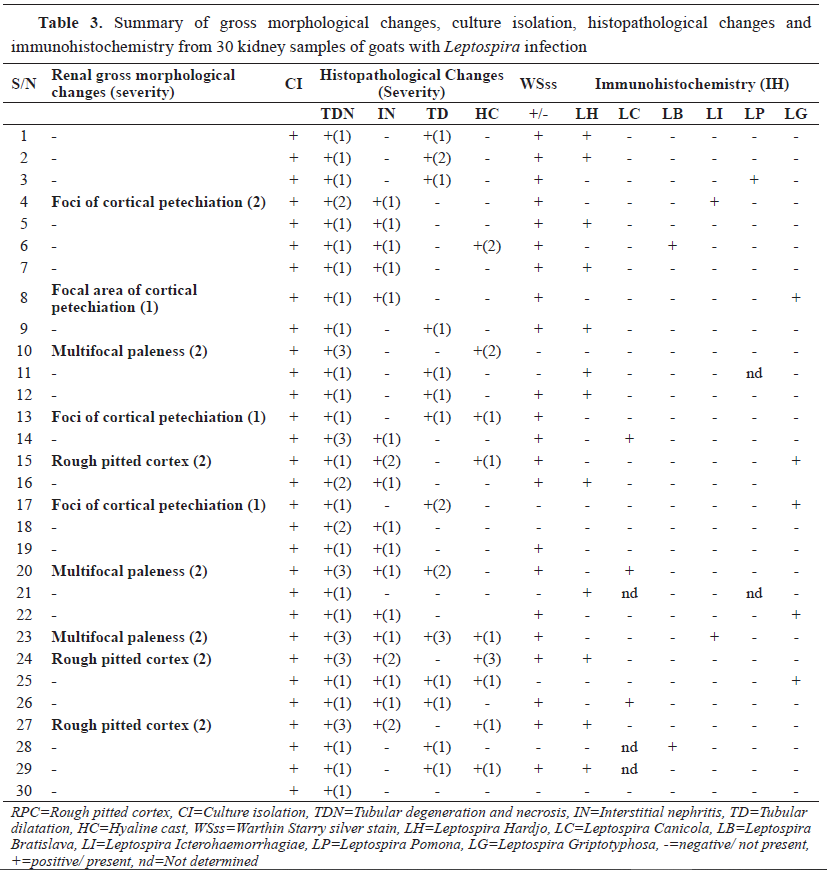
Three kidney sections were positive on IS, but negative on WSs and IH, and two were IS and WSs positive but IH negative. Immunoreactivity was restricted to the proximal distal convoluted tubules and collecting ducts. Renal sections without specific monoclonal antibody were negative (
Fig. 5d).
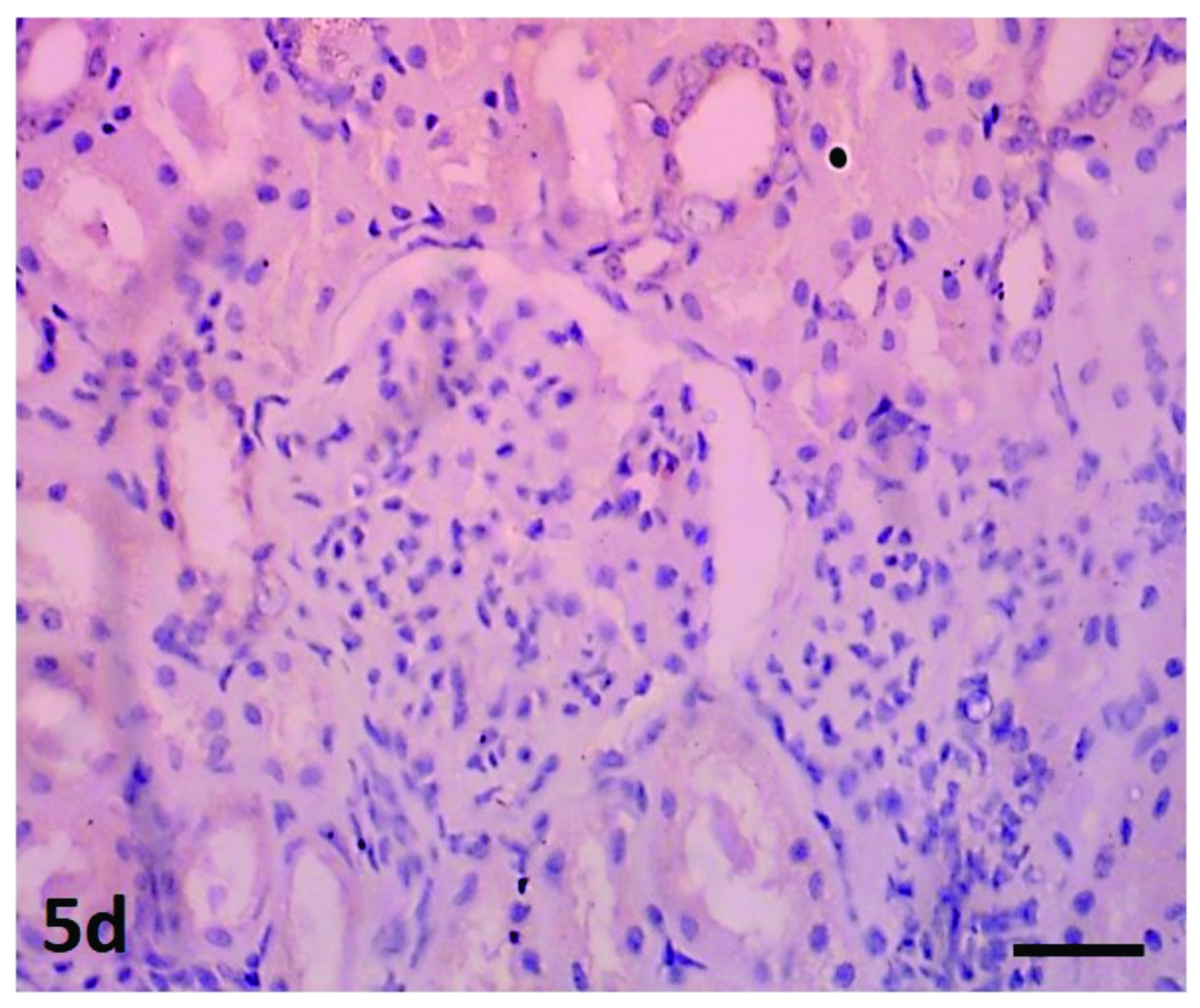 Figure 5d.
Figure 5d. Kidney section without specific monoclonal antibody showing negative immunoreactivity. Streptavidin-biotin immunoperoxidase; x400, Bar=150μm
DISCUSSION
This study demonstrated
Leptospira infection in goats slaughtered in the two abattoirs in southwest Nigeria. This is evidenced by the high prevalence of 51.5% of
Leptospira infection using the EMJH medium when compared with a serological survey in the same area more than 17 years ago (
13). In their study, 13.1% of 320 goats were serologically positive to
Leptospira infection in Ibadan, Oyo state, Nigeria. The prevalence in this study was lower than that found in a previous study in the Northern part of the country, where 72.0% seroprevalence was reported (23). In countries such as Iran, Brazil, Belize and Egypt, the seroprevalence for leptospirosis in goats was found to be lower (1.2%, 42.0% and 42.1% respectively) (
24,
25,
26), while in India and New Zealand was higher (55.2% and 70.0%, respectively) (
27,
28) than the results of our study. This might be due to variations in environmental factors such as incessant rainfall, flooding, higher environmental temperatures and host vectors which facilitate
Leptospira dissemination (especially rodents) and infections (
29). It is also possible that the differences in prevalence in these regions of the world might be due to variations in control and diagnostic strategies. It is noteworthy to mention that the assessment of the seroprevalence of leptospirosis in goats from these parts of the world can only suggest exposure of goats over a certain extent of time but not necessarily at the moment of sampling. The current study suggests ongoing infection which was confirmed by the isolation of
Leptospira organisms from the kidney of these goats. The area of sample collection and the number of samples in this study might not be appropriate for precise determination of the leptospirosis prevalence, however, the presence of certain serovars in this region was determined. This finding will be useful to abattoirs workers, veterinary practitioners and farmers who are highly exposed to this disease with zoonotic implications. There was no significant influence of sex, breed and location of goats on the prevalence of leptospirosis, but there was a significant difference in
Leptospira infection in animals between the age of 1-2 years. The high prevalence of leptospirosis in this age group might be due to a lack of immunity since goats are rarely vaccinated against leptospirosis in the study area.
The macroscopic lesions observed in this study are consistent with a previous report in cattle (
28). No relationship existed between macroscopic lesions and the presence of the infection because most of the samples that showed no gross morphological changes were positive for leptospirosis by IS and IH.
The most consistent histopathological changes observed in all the goats were necrosis of tubular epithelium (67.4%), interstitial lymphoplasmacytic infiltration (61.8%) and protein casts (61.3%). This is in agreement with previous studies in cattle and wild animals (
29). However, Skilbeck et al (
30) did not observe significant histopathological changes in the kidneys of cattle which were positive on IS.
Small ruminants have been regarded as accidental hosts of
Leptospira of various serovars (
31). These serovars common for most domestic and wild animals were not specific for the analyzed specimens (
31). Several studies have shown that
Leptospira infection in goats and sheep are common and that they can act as the potential disseminators of the organism into the environments (
32,
33). However, they have been usually regarded as incidental hosts (
31). In small ruminants, serovar Hardjo subtype Bovis is considered to be common (
34). In this study, despite the fact that the monoclonal antibody of
L. Hardjo subtype Bovis was not used,
L. Hardjo subtype Prajitno was the most predominant serovar with a detection rate of 48% (12/25) using IH. Previous studies have shown that serovar Hardjo subtype Prajitno has higher virulence and zoonotic implications than the serovar Hardjo subtype Bovis (
35,
36). It is also possible that cross-reactivity between these subtypes might have occurred in this study. Grippotyhphosa (5/25, 20%), Canicola (3/25, 12%), Bratislava (2/25, 8%), Icterohaemorrhagiae (2/25, 8%) and Pomona (1/25, 4%) have consecutively lower virulence. The positive confirmation of these serovars in the study area is important for planning preventive measures for animal handlers, farmers, abattoir workers, and consumers.
The specificity and reliability of the WSs impregnation method for the detection of
Leptospira in this study was low. This was confirmed by the finding of 5 kidney samples which were positive on IS and IH, but negative on WSs impregnation (
Table 3). This is in agreement with previous studies (37). Moreover, kidney sections 2 and 3 which were IS and WSs positive had negative immunoreactivity. This finding suggests that the infecting serovars were not included in monoclonal antibody cocktails. With the ongoing molecular characterization of these isolates, we believe that new serovars might be detected in this animal species.
Over the years, most of the diagnosis of leptospirosis in small ruminants has relied solely upon serological assays (
29,
38), whereas IS, WSs and IH are rarely performed (
39). The authors of this study could not find any previous work in which IH had been used as a diagnostic tool for caprine leptospirosis. To the best of our knowledge, this appears to be the first report in which IH was used to detect
Leptospira antigens in the renal tissues of goats, and this greatly enhanced the specific diagnosis of the disease and the determination of the various serovars involved. IH employed in this study enabled
Leptospira serovar determination, antigen detection, localization and distribution within the renal tissue. This is consistent with the findings of Rosetti et al. (
21) who reported that IH yielded more serovar-specific positive results than other diagnostic methods, since WSs impregnation and isolation could only detect the presence of the organism without serovars’ determination.
CONCLUSION
In conclusion, this study revealed a high prevalence of caprine leptospirosis in the study area (51.5%) using EMJH medium compared with previous studies in which serology was used as the only diagnostic tool. IS and WSs proved to be useful diagnostic tools in that they revealed evidence of infection, but IH proved to be effective in identifying most of the various serovars involved in the study area. Consequently, this study would create an awareness of the zoonotic implications of caprine leptospirosis and assist policymakers to develop control strategies for the control of leptospirosis in goats and humans in this region.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest related to this article.
ACKNOWLEDGEMENTS
The authors of this manuscript would like to offer their profound gratitude to Mr. James of the Department of Veterinary Pathology, Federal University of Agriculture, Abeokuta Nigeria for his technical help. We are also indebted to Prof. Hartkreel of the Royal Tropical Institute, Amsterdam, the Netherlands who graciously offered the monoclonal antibodies used in this study.
AUTHORS‘ CONTRIBUTIONS
A.O.L. was the project leader, initiator and the pathologist that carried out the Warthin Starry silver stain, immunohistochemistry, read the slides and contributed immensely to the write up of the manuscript. A.R.E. was project leader as well, initiator and the pathologist that contributed to the slide reading and write up of the manuscript. O.O.E. and T.M.O. were involved in the collection of the kidney samples from different areas and laboratory works. O.M.O. was the pathologist who contributed to the slide reading and write up of the manuscript. A.O.J. did the statistical analysis and provision of figure one.

 10.2478/macvetrev-2020-0031
10.2478/macvetrev-2020-0031









