Diarrhea in canines is one of the most common problems small animal practitioners face with today, and bacterial enteropathogens play a vital role in many of these cases (
1). Clinical signs can differ among moderate, self-limiting diarrhea, and an acute, potentially fatal, diarrheal syndrome (
2). Pathogenic
Escherichia coli,
Clostridium perfringens,
Clostridium difficile,
Campylobacter spp. and
Salmonella spp. are the most commonly reported canine diarrheal bacteria (
2).
Escherichia coli (
E. coli), a member of the family
Enterobacteriaceae, is part of a normal animal and human commensal bacterial flora (
3,
4).
E. coli is strongly involved in clinical cases of dog diarrhea (
3). Diarrheagenic
E. coli isolate may belong to the enteroinvasive
E. coli (EIEC), enterohaemorrhagic
E. coli (EHEC), enteropathogenic
E. coli (EPEC), entroaggregative
E. coli (EAEC), necrotoxic
E. coli (NTEC), enterotoxigenic/shiga-like toxinproducing
E. coli (STEC) or diffusely adherent
E. coli (DAEC) strain/pathotypes, depending on the type of virulent gene(s) being formed and the type of lesion-induced (
3,
5).
Canine diarrhea may not be caused primarily by
E. coli, while pathogenic
E. coli strains have been widely incriminated in human and animal cases of diarrhea (
6). Numerous infective (canine distemper, parvoviral enteritis, coronavirus infection, and helminthiasis) and non-infective conditions in dogs include alterations in intestinal mucosa consistency resulting in enteritis and diarrhea (
7). Under these conditions, pathogenic
E. coli is being released by the feces and may cause immune depression.
E. coli strains have been documented to harbor genes that encode virulent pathogenic factors (
5,
6). Virulence factors which are often present in pathogenic
E. coli strains are used for the classification of different pathotypes (
stx1, stx2, cnf1, cnf2, and
eae) (
3). These virulent factors were widely reported to be associated with human and animal diarrhea cases (
8).
The use of antibacterial agents in dogs (fluoroquinolones, potentiated sulfonamides, penicillins, cephalosporins, chloramphenicols, tetracyclines, and aminoglycosides) in suspected cases of bacterial infection is often performed by veterinary clinicians and non-veterinarians, particularly in countries where there are no strict regulations for the use of these drugs (
7,
9,
10). This led to increased identification of antimicrobialresistant
E. coli, both pathogenic and nonpathogenic strains, in the companion animals worldwide (
9,
11).
E. coli develops resistance as a result of prolonged exposure to antibacterial agents, particularly when applied in subtherapeutic doses, and by acquiring antibacterial resistance genes from other commensals or transient pathogens that colonize the body or the environment (
4,
12,
13). Shedding fecal
E. coli is an important source of environmental contamination by companion animals. Animals with clinical manifestations of diarrhea usually suffer from immune suppression which favors increased fecal shedding of
E. coli (
15). Such animals defecate frequently and uncontrollably thus spreading the bacterium (
13). Moreover, pathogen presence in dog’s diarrheal feces poses a serious threat to public health after zoonotic transmission. Dog owners/trainers, children, and veterinarians are at higher risk as a result of handling and close contact with these animals (
16).
Isolation and identification of antimicrobialresistant diarrheagenic
E. coli have been reported from dogs with or without diarrhea, and their handlers in Europe, America, Asia and North Africa (
12,
13,
15,
17,
18). Nevertheless, there is comparatively less knowledge in West Africa about the prevalence and antimicrobial resistance of
E. coli isolates in diarrheal dogs, which was the main motive for our investigation.
MATERIAL AND METHODS
Study areaThe present study was conducted in Maiduguri Metropolis, Borno State Nigeria. This area lies within the semi-arid zone of the North-Eastern part of Nigeria (
19). Borno State is situated between Latitude 11̊ 46’ 18’’N – 11̊ 53’ 21’’N and Longitudes 13̊ 02’ 23’’E – 13̊ 14’ 19’’E (20, 21, 22) (
Fig. 1).
 Figure 1.
Figure 1. Map of the studied area (asterisk). Source: Google map
Study populationRectal swab samples were randomly collected from diarrheic dogs in Magaram and Sabon layi wards, including Elkanemi Park in Maiduguri. Samples were collected from June to September 2019. Dogs were categorized based on breed, age, sex, and management system (owned or stray). Dogs from 0-12 months were considered as puppies, while above 12 months as adult dogs.
Sample collection
Two hundred rectal swab samples were collected using sterile swab sticks (Oxoid, UK). All the samples were labeled with an identification number and date of collection and immediately transported on ice-pack to the Department of Veterinary Medicine Diagnostic Research laboratory, University of Maiduguri for analysis.
Bacteriological examinationThe fresh rectal swab samples were inoculated onto MacConkey agar (MCA, HiMedia, Mumbai, India) applying the quadrant streak plate method for pure colony isolation. Each plate was labeled accordingly, and thereafter incubated aerobically at 37 °C for 24 hours. The pink (lactose fermenting) colonies (
Fig. 2) were selected and subcultured onto Eosin Methylene Blue agar (EMB, HiMedia, Mumbai, India) and incubated at 37 °C for 24 hours to obtain a pure strain culture. Subcultured strains from MacConkey agar, which appeared as smooth, glossy, pinkish, with a greenish metallic sheen on EMB (
Fig. 3), were presumptively considered as
E. coli. These strains were further confirmed as
E. coli by Gram staining, and standard biochemical tests (Indole, Methyl-Red, Voges-Proskauer, Citrate utilization, and Urease test). All confirmed isolates were streaked on a nutrient agar slant and stored at 4 °C for further use.
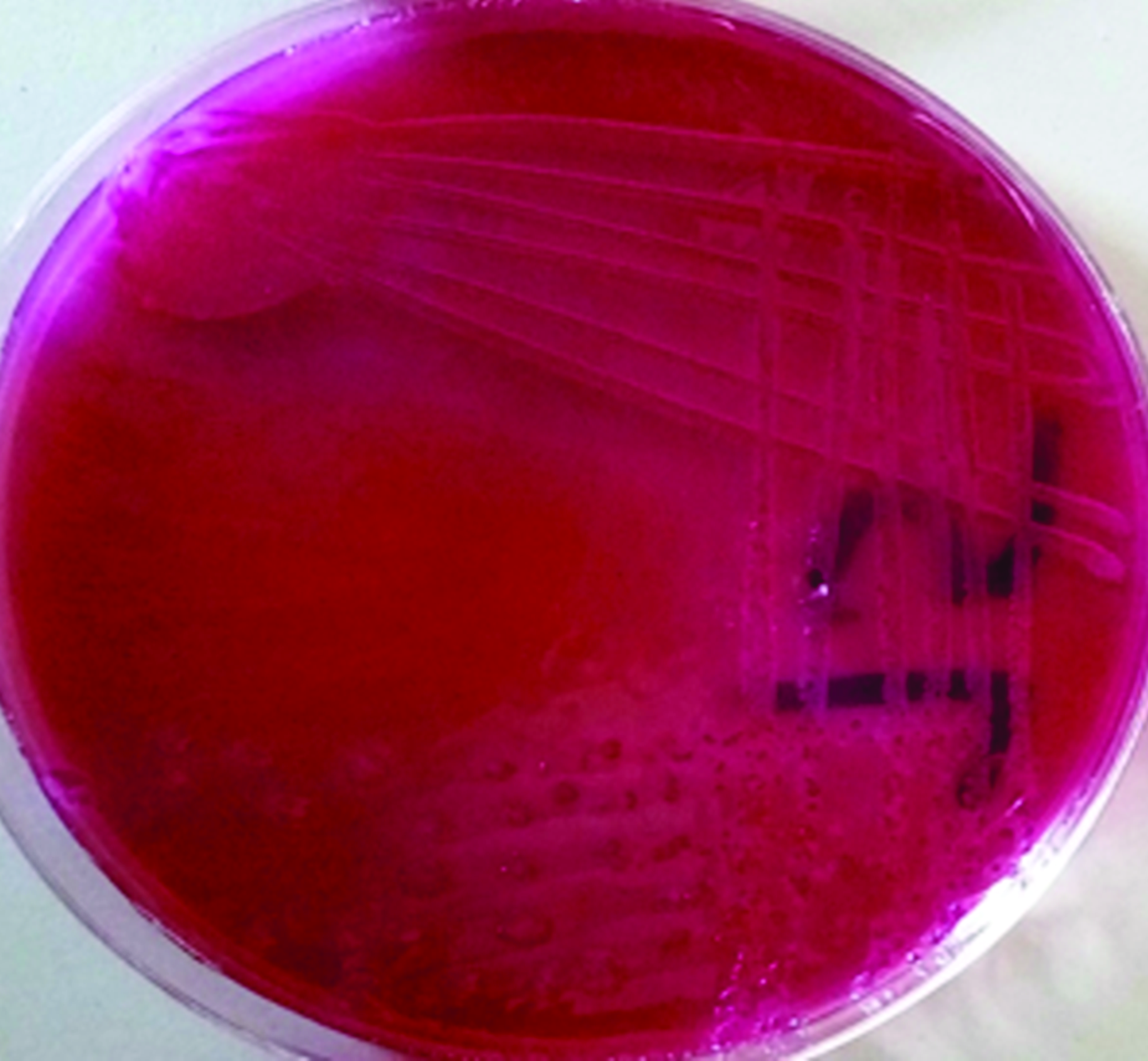 Figure 2.
Figure 2. A typical pinkish colonies of positive isolate on MacConkey Agar
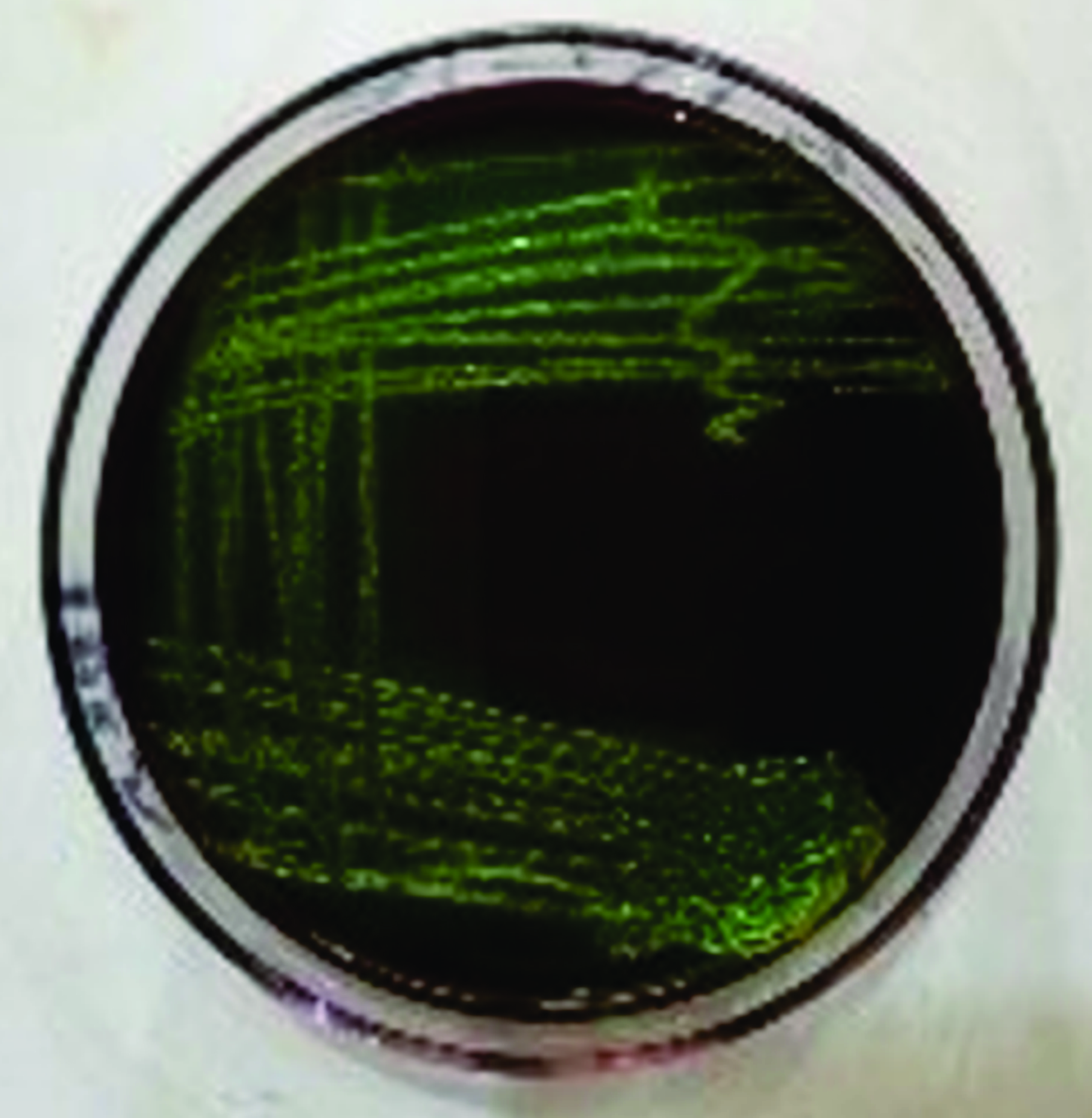 Figure 3.
Figure 3. An intense blue-green metallic sheen colouration of
E. coli on Eosin Methylene Blue (EMB) agar
Antimicrobial susceptibility testing The antibiotic susceptibility testing of the isolates was carried out using the disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (
23). Briefly, wellisolated colonies were transferred into a test tube of brain heart infusion broth (BHI, HiMedia, Mumbai, India) and incubated for 2-5 hours at 37 °C to obtain turbidity equivalent to 0.5 McFarland standard. By sterile cotton swab stick, the broth culture was then evenly spread over the surface of Mueller Hinton agar plates (HiMedia, Mumbai, India). The strip with multiple antibiotics was placed on the agar and gently pressed with sterile forceps to obtain uniform close contact with the medium. The antibiotics used in the present study were: Nitrofurantoin (N, 100 μg), Gentamicin (GN, 10 μg), Ciprofloxacin (CIP, 10 μg), Chloramphenicol (C, 10 μg), Ofloxacin (OF, 10 μg), Cefuroxime (CF, 30 μg), Pefloxacin (PF, 10 μg), Ceftriaxone (CT, 30 μg), Amoxicillin (AX, 30 μg) and Streptomycin (ST, 30 μg) (Polytes Medical Laboratories, Enugu Nigeria). The plates were then incubated aerobically at 37 °C for 24 hours. The diameter of each zone of inhibition (in mm) was measured. The interpretation of the measurement was described as sensitive (S), intermediate (I), and resistant (R), according to the CLSI manual (
23).
RESULTS
Out of 200 rectal swab samples, 147 (73.5%) were positive for
E. coli. The highest number of positive samples were detected in Elkanemi Park (90%), followed by Magaram (71%) and Sabon gari (65%) (
Table 1).
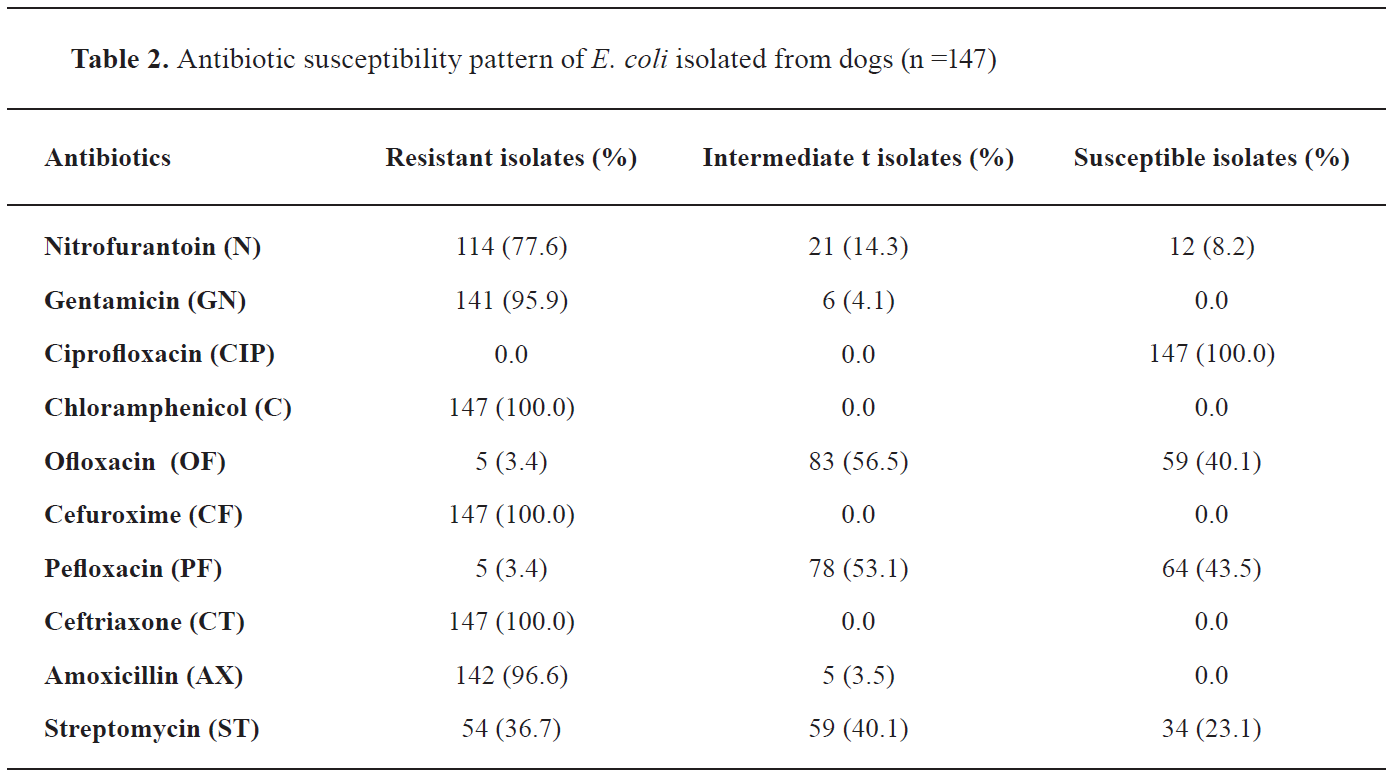
 Antimicrobial susceptibility profile of the E. coli
Antimicrobial susceptibility profile of the E. coli The result of antimicrobial susceptibility tests (
Table 2 and
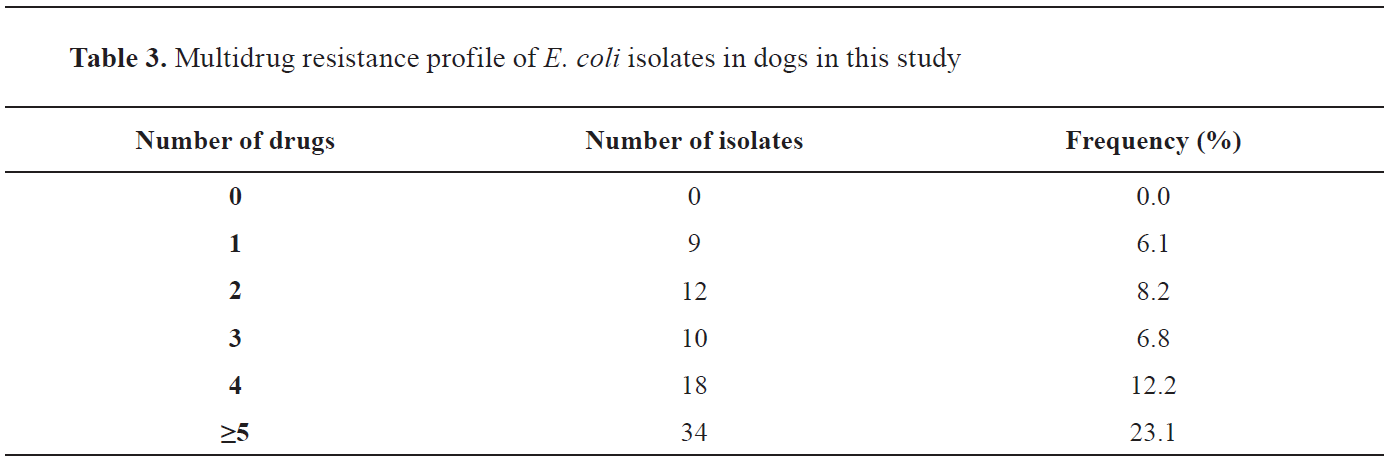
Fig. 4) have shown high resistance to Chloramphenicol (147 isolates, 100%), Cefuroxime (147 isolates, 100%), Ceftriaxone (147 isolates, 100%), Amoxicillin (142 isolates, 96.6%), Gentamicin (141 isolates, 95.9%) and Nitrofurantoin (114 isolates, 77.6%), while the isolates were highly susceptible to Ciprofloxacin (147 isolates, 100%). Multiple antibiotic resistance was detected in all
E. coli isolates, as shown in
Table 3.
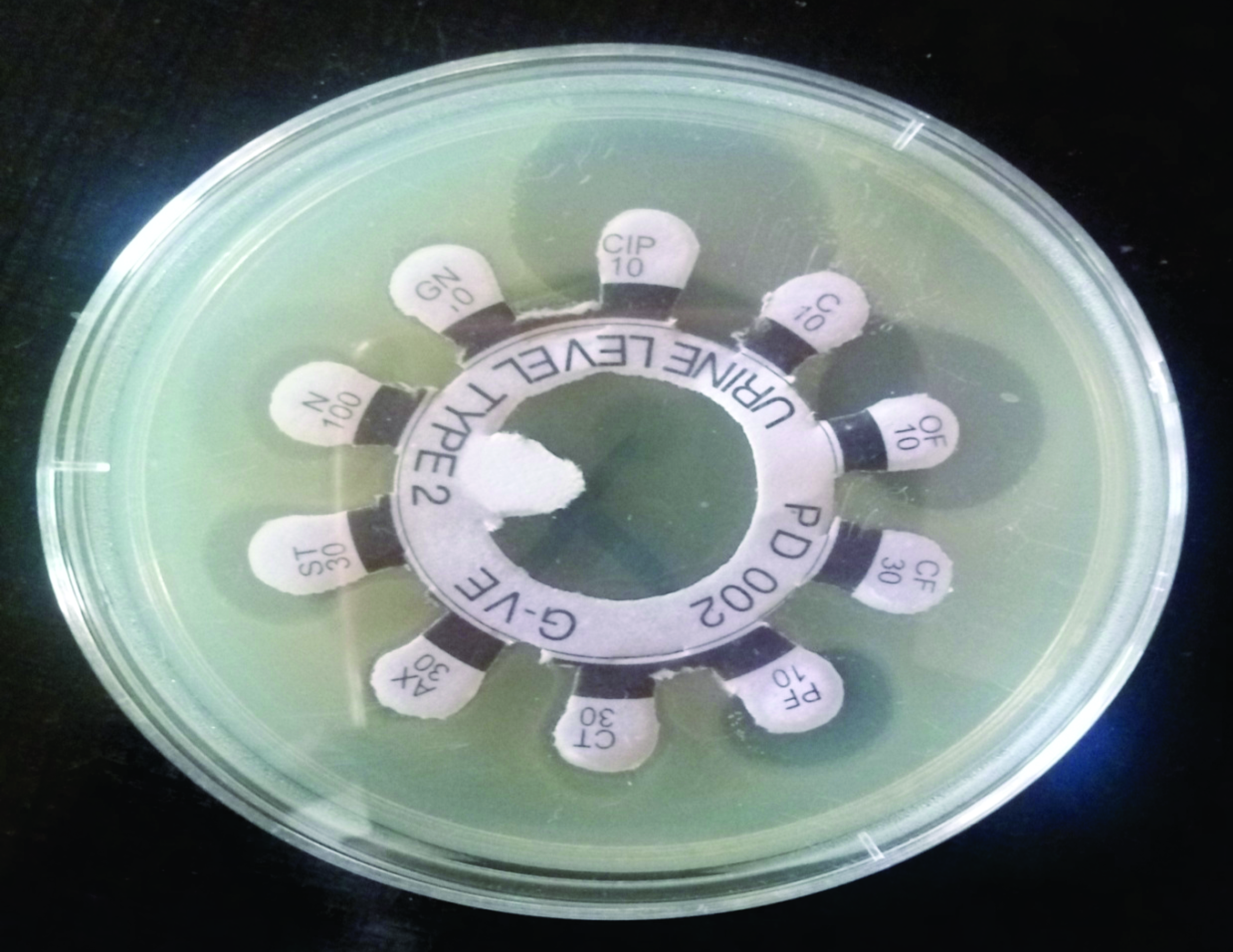 Figure 4.
Figure 4. Antibiotic susceptibility pattern of
E. coli isolates showing various zones of inhibition.
DISCUSSION
The overall isolation rate of
E. coli from diarrheic dogs in this study was very high (73.5%), most likely as a consequence of the interaction between the pets and stray dogs which increased the potential for pathogenic
E. coli transmission and spreading among the investigated animals.
These findings concurred with the study carried out by Puno-Sarmiento et al. (
3). This high prevalence of
E. coli (73.5%) in both categories of diarrheic dogs (puppies and adults) sampled from different areas within Maiduguri metropolis (Magaram, Sabon layi and Elkanemi Park) is in agreement with the results of Yousif et al. (
24), who reported a high prevalence of E. coli (83%) in adult dogs and puppies, and with the results of Hassan (
27) reporting high prevalence in both diarrheic categories (68.75%) and low prevalence rate (7.95%) in non-diarrheic categories. The significance of
E. coli pathogenicity has been confirmed with the findings of Wang et al. (25) reporting that all dogs developed acute watery or mucous diarrhea on the second day of infection, and a study from Brazil where out of 154 stool samples tested, 37 (24%) were positive for
E. coli strains, harboring at least one virulence gene detected in PCR analysis (
26).
In the current research, the
E. coli prevalence rate recorded in Elkanemi Park was higher than that recorded in Magaram and Sabon Gari. This may be due to the feeding habits of dogs in this area, because they are typically fed food leftovers found in dumpy areas across the ward. Foodborne transmission is the most important common means of infection (
28), especially in places contaminated by feces which is containing
E. coli (
14).
The antimicrobial susceptibility pattern of
E. coli isolates recorded in the present study established a high level of resistance to Chloramphenicol, Cefuroxime, Ceftriaxone, Amoxicillin, and Gentamicin. The
E. coli isolates were highly susceptible to Ciprofloxacin, and this could probably be due to the infrequent use of this drug in the studied area. This is in agreement with Aslani et al. (
29), who reported that only a few isolates were resistant to ciprofloxacin, and the results of Grave et al. (
30), where the high sensitivity was shown only to ciprofloxacin among the used antimicrobials. Antimicrobial resistance in bacteria is a phenomenon that has been in constant evolution since the introduction of antibiotics. Several factors are known to promote bacterial resistance, including failure to follow the treatment regimen, prophylactic use of antibiotics, and the use of antibiotics as growth promoters. Fluoroquinolones are newer drugs that are relatively expensive, and therefore less available for use and potential abuse in the study areas. Therefore, the infrequent use of this drug in these areas is probably the main reason for high susceptibility to Ciprofloxacin of isolated
E. coli strains.
However, our results are incompatible with the findings by Younis et al. (18), who reported that
E. coli isolates were sensitive only to amikacin. These reports and the findings of the present study indicate different resistance patterns of
E. coli.
Multidrug resistance is a condition in which bacteria are resistant to a specific drug in three or more antimicrobial classes (
30,
31). Multidrug resistance to different antibiotics recorded in this study corresponds with the findings of Puno-Sarmiento et al. (
3), who reported multiresistant strains in most diarrheic
E. coli strains to both the frequently and less-frequently used antimicrobials.
Most dog owners seek out for the aid of a veterinarian only when their pets display overt disease signs, which is especially common in developing countries where most dog owners rarely carry out preventive programs. Normally, in rural and suburban areas the level of compliance may be even more reduced. However, a considerable number of dog owners are increasingly aware of the risks and potential costs related to the nonadoption of preventive measures directed to avoiding exposure to certain pathogens, such as
E. coli. Thus, it is fundamental that veterinarians guide dog owners towards the establishment of long-term preventive programs against infectious diseases including those caused by
E. coli.
CONCLUSION
The results of the present study confirmed the presence of
E. coli in 73.50% dogs with diarrhea in Maiduguri area. The highest number of positive samples was obtained in Elkanemi Park (90.00%), compared to Magaram (71.42%) and Sabon gari ward (65.00%). All isolates shown antimicrobial resistance to Chloramphenicol, Cefuroxime, Ceftriaxone, Amoxicillin, and Gentamicin. However, Ciprofloxacin has shown 100% antibacterial efficiency against obtained
E. coli isolates, which indicates to be a first choice of antimicrobial for the treatment of dogs with diarrhea in the studied areas. Furthermore, it is important to adopt and apply guidelines for the correct use of antimicrobials in small animal practice to reduce the emergence of multidrug resistance among
E. coli in companion animals.
CONFLICT OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTS
We thank Mal. Isa Adamu Gulani for expert technical assistance.
AUTHORS’ CONTRIBUTION
MM, YA, YMBK and LA planned and designed the experiments. MM carried out the study and KUE, JUA, JRL, and AGB provided technical support. MM and LA carried out statistical analysis. All authors read and approved the manuscript.

 10.2478/macvetrev-2020-0035
10.2478/macvetrev-2020-0035






