Pregnancy-specific protein B (PSPB) is produced by mono and binucleate trophoblast cells in the placenta of ruminants during pregnancy. This study was designed to determine the pattern of serum PSPB in Yankasa ewes during pregnancy and postpartum periods. Mature cycling Yankasa ewes were synchronized and divided into two groups A (n=11) and B (n=13). Group A was bred, while group B was unbred. Blood samples for PSPB assessment were collected from the ewes starting from the day of breeding until 4 weeks post-lambing. All pregnant Yankasa ewes lambed with singleton lambs after an average of 151.18 days. There was a significant (p<0.05) increase in PSPB in pregnant compared with the non-pregnant ewes in the period between 3 weeks post-breeding and 3 weeks post-lambing. Peaks were detected in the first (100.60 ng/ml), second (133.90 ng/ml), and third (114.82 ng/ml) trimesters at 5, 10 and 21 weeks of gestation, respectively, but steadily decreased within 4 weeks (2.38 ng/ml) postpartum. In conclusion, PSPB detected pregnancy in Yankasa ewes from 3 weeks post-breeding with peak levels at 5, 10 and 21 weeks post-breeding in the first, second, and third trimesters, respectively. PSPB decreased gradually after lambing until 4 weeks postpartum.
Sheep are estimated to make up 27% (37.4 million) of Nigeria`s livestock population (
1), where they are kept for meat and milk production (
2). They also play a socio-economic role in Nigerians’s life, particularly in the northern part where they are kept and used during festive periods such as
Eld-el Kabir, wedding, and naming ceremonies (
3). Sheep are part of almost all farming systems in Nigeria, where they are associated with mixed farming and pastoralism (
4). They are essential in sustaining low-income families, mainly in the rural areas of Nigeria (
5). Generally, four breeds of sheep are indigenous to Nigeria, namely Balami, Uda, West African dwarf (WAD) and Yankasa. However, Yankasa sheep is the most abundant breed in Nigeria (
2), females attaining puberty at 238±23.4 days at an average weight of 18.4±0.4 kg (
6). Adult Yankasa ewes weigh 21.48±0.48 kg, and produce lambs in a singleton to twin ratio of 1:2.8 (
7). Pregnancy is the period beginning immediately after fertilization from a fertile mating till parturition (
8) and takes about 147–152 days in sheep (
9). It is an integral part of animal reproduction, characterized byembryo formation and fetal development due to changes in the physiology of the reproductive tract (
8,
9). During this period, several biomarkers, such as enzymes, growth factors, inhibitors, and hormones are released into the circulation (
10). Pregnancy specific-protein B (PSPB) is produced during pregnancy in sheep and can be considered as its marker (
11).
PSPB is also known as pregnancy-associated glycoprotein (PAG), pregnancy-serum protein 60 kDa, and SBU-3 antigen (
10). They constitute a large family of placenta glycoproteins, belonging to a group of proteolytic enzymes known as aspartic proteinases (
12). These proteins are produced by mono and binucleate trophoblast cells in the ruminant placenta and then released into the maternal circulation. Its levels in plasma and milk are used for pregnancy diagnosis (
13), fetal number assessment (
14), pregnancy wastage (
15,
16), pregnancy loss (
14), and obstetric diseases (
17). The concentration of PSPB is influenced by animal species, breed, parity, and sex of the fetus (
18,
19). The pattern of PSPB during pregnancy and postpartum periods has been described in some breeds of sheep, such as Churra, Merino (
18) and Assaf (
20). They have also been described in Boer (
21), Moxoto and Canine goats (
22), as well as in buffalo cows (
23). However, the pattern of PSPB during pregnancy and postpartum periods in Yankasa ewes has not been described to the best of our knowledge. Information on the profile of PSPB in pregnancy will assist in planning feeding strategies to avoid metabolic diseases related to pregnancy, such as pregnancy toxemia and birth of viable and healthy offsprings. In addition, it will contribute to the existing knowledge on the use of PSPB in pregnancy diagnosis and monitoring improving animal productivity and reproductive performance.
MATERIAL AND METHODS
Experimental siteThe study was carried out at the animalhouse of the Faculty of Veterinary Medicine and Reproduction Laboratory of National Animal Production Research Institute (NAPRI), Ahmadu Bello University, Zaria, Nigeria. Zaria is within the Guinea Savannah zone of Nigeria, between 110 and 120 North and between 70 and 80 East at 650 m above sea level.
Experimental animalsThirty cycling Yankasa ewes aged between 1.5 and 2.5 years, with an average weight of 19±1.14 kg were used for this study. The animals were acquired from the small ruminant flock of NAPRI and local markets around Zaria, Nigeria. They were acclimatized for 6 weeks and screened for common parasites during this period. The animals were fed on hay and concentrate supplements in the morning and evening, whereas water and salt-blocks were given
ad libitum. Ethical approval for this study was obtained from the Ahmadu Bello University Committee on Animal Use and Care, with approval number ABUCAUC/02/2013/001.
Experimental designFollowing acclimatization, estrus synchronization was achieved using 5 mg of Lutalyse® (Dinoprost tromethamine). Twenty-six ewes showed signs of estrus. Thirteen out of the twenty-six ewes were bred with rams of good fecundity (sperm volume 0.72 ml, sperm concentration – 300x106; percentage of live sperm 97.65%; sperm abnormalities - 20%), while the remaining ewes were unbred. Pregnancy was determined by non-return to estrus and persistent high blood levels of progesterone (P4) 18-24 days post-breeding. The bred and unbred ewes were tagged and grouped as A and B, respectively. On day 45 post-breeding, two ewes in group A were diagnosed non-pregnant by ultrasonography (Medison SA600V, Germany) and subsequently excluded from the study. Blood samples were collected on a weekly basis (7±1 day) in the period between the onset of breeding and four weeks post lambing in ewes of both groups.
Blood collection and analysisAbout 5 ml of blood was collected weekly from the jugular vein of each ewe into plain test tubes and centrifuged at 9,000 g for 5 mins. Blood serum was harvested and stored at -20 oC until analysis.
Assay for pregnancy-specific protein BEnzyme-linked immunosorbent assay was carried out on the serum samples using bioPRYN quantitative PSPB ELISA kits (Biotracking LLC; Moscow ID) to determine the concentrations of PSPB in the test samples. The manufacturer`s instructions were followed, as previously described by Adeyeye et al. (17). Values of obtained optical density were added into an Excel package pre-programmed by the manufacturers, from where actual PSPB values were obtained. The test kit had a sensitivity of 99.9% and a specificity range of 90-99%.
Data analysisData of PSPB values were analyzed using t-test of GraphPad (2000) and presented as mean ± error of mean in graphs. Values of
p<0.05 were considered statistically significant.
RESULTS
All ewes in group A lambed singleton lambs after a mean ± standard error gestation length of 151.18±0.58 days. The profile of PSPB during pregnancy is presented in
Fig. 1. The mean weekly profile of PSPB ranged from 0.27 to 133.90 ng/ml and 0.10 to 0.97 ng/ml in pregnant and non-pregnant ewes, respectively. There was no significant (
p>0.05) difference in serum PSPB levels between pregnant and non-pregnant ewes from the time of first standing heat to the second-week post-breeding. However, serum PSPB significantly (
p<0.05) increased in pregnant ewes compared to non-pregnant ewes in the period between the third-week and the time of parturition. In the first trimester, PSPB levels peaked at the fifth-week post-breeding with a mean of 100.60 ng/ml, while the peak in the second trimester occurred at the tenth-week post-breeding with a mean of 133.90 ng/ml. There was a gradual increase in PSPB levels in the third trimester reaching the peak a few days before the parturition.
 Figure 1.
Figure 1. Pattern of pregnancy-specific protein B (PSPB) during pregnancy in Yankasa sheep Mean ± SEM gestation length of pregnant ewes - 151.18±0.58
*Statistically significant (p<0.05) from week 3 until lambing
The serum PSPB levels of postpartum ewes are presented in
Fig. 2. The mean PSPB levels post-breeding ranged from 2.38 ng/ml at the fourth-week to 114.82 ng/ml at parturition. There was a gradual and statistically significant (
p<0.05) decrease in PSPB between postpartum and nonpregnant ewes from lambing until the third week postpartum. The serum levels of postpartum ewes were not statistically significant (
p>0.05) at the fourth-week postpartum when compared with non-pregnant ewes.
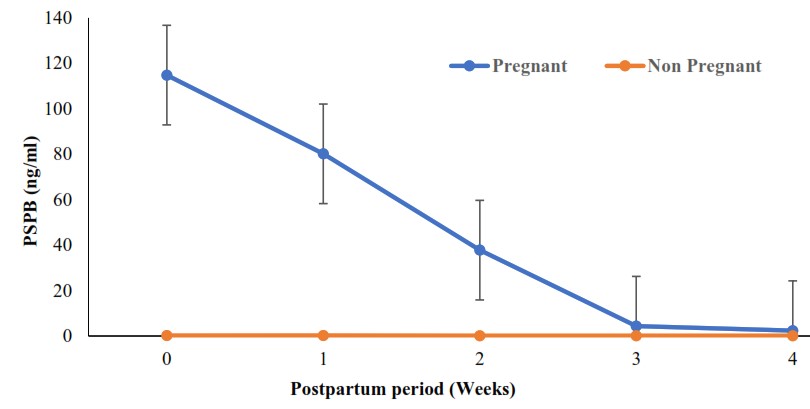 Figure 2.
Figure 2. Pattern of pregnancy-specific protein B (PSPB) during postpartum period in Yankasa sheep
*Statistically significant (
p<0.05) from lambing until week 3 postpartum
DISCUSSION
PSPB is a biomarker synthesized in the superficial layer of the developing trophoblast of ruminants and released into the maternal circulation (
10). Its concentrations are detected and used to investigate placental function during pregnancy (
11). The present study describes the pattern of PSPB during pregnancy and postpartum periods in Yankasa sheep for the first time, which is the most abundant breed of sheep in Nigeria (
2). There were no multiple births among the Yankasa ewes used in this study. PSPB increases in ewes with multiple births (
13) as a result of the increase in the total weight of placentomes that rises with the fetal number (
24).
In our study, the levels of PSPB in pregnant ewes were not significantly different from those in non-pregnant ewes within two weeks of breeding. However, it increased substantially between weeks 2 and 3 (days 14 and 21) post-breeding. This finding is similar to previous reports in other breeds such as Merino (
18), Suffolk, Panama (
25), Blackheaded German Mutton, Rhoen and Dopper ewes (
26). It is also similar to previous reports in goats (
20,
27) and buffaloes (
23). Substantial PSPB levels between weeks 2 and 3 may suggest an early differentiation of binucleated cells in the trophoblast, movement from the placenta to the maternal endometrium and subsequent implantation in Yankasa ewes. The flow of binucleated cells to the endometrium is associated with the appearance of PSPB in maternal circulation during pregnancy (
18,
25,
28). The studies of El-Amiri et al. (
28) further showed that PSPB is specifically detectable by day 18 of gestation in pregnant ewes but is low or undetectable in the non-pregnant ewes. This hypothesis needs to be evaluated in Yankasa ewes and other Nigerian breeds of sheep to determine the exact period between days 14 and 21 when PSPB can be used for pregnancy diagnosis. Early pregnancy diagnosis in sheep is crucial because it assists farmers in separating non-pregnant ewes for rebreeding.
The PSPB profile of pregnant Yankasa ewes in this study steadily increased with an uncomparable difference with the non-pregnant ewes from week 3, reaching the first peak at week 5. This finding is similar to earlier reports of Ranilla et al. (
18) in Merino ewes, Willard et al. (
25) in Suffolk and Panama ewes, as well as the report of Ledezma- Torres et al. (
26) in Blackhead German Mutton and Rhoen ewes. It is also similar to the report of Batalha et al. (
22) in Alpine goats but varies with the report of Ranilla et al. (
18) in Churra ewes. This may be associated with increased binucleated cell production following early differentiation. The highest peak in the first trimester was at week 5, similar to the pattern in Merino ewes but dissimilar to those in Churra ewes (
18). This could be associated with variation in the genetic make-up of the breeds. Previous studies suggest a difference in PSPB levels among different breeds (
22). Roberts et al. (
19), therefore, recommended PSPB standards to be determined for every ovine breed. There was a slight decrease in PSPB of pregnant Yankasa ewes from week 5 to 7 post-breeding, and this varied with previous reports in Churra ewes (
18) and Alpine goat (
22). However, it is similar to the report of Ranilla et al. (
18) in Merino ewes. The decline may be associated with the period of genes switching on and off. PSPB is a group of related proteins whose expression varies during pregnancy.
The PSPB of pregnant Yankasa ewes displayed a progressive increase from week 7, attaining the highest peak of the second trimester at week ten post-breeding. Progressive increase similar to this has been reported in Blackheaded German Mutton, Rhoen, Dopper (
26), Churra and Merino ewes (
18). However, their peaks were at week 9 post-breeding, probably due to breed variations. The second trimester peak of PSPB at week 10 was the highest during pregnancy in our study. This has also been reported in Corriedale and Ile de France cross ewes (
29), Boer goats (
21) and Indian native goats (
13), although their peaks were at day 95 (week 13-14), day 60 (week 8-9), and day 51 (week 7-8) post-breeding, respectively. The high level of PSPB during the second trimester may be associated with the numerous placenta proteins produced during this period. El Amiri et al. (
30) and El Amiri et al. (
31) had previously isolated several proteins between days 60 and 100 post-breeding in sheep. Several placenta proteins are also produced during the second trimester in cattle (
32), and there is evidence of structural and immunological similarity between PSPB in sheep and cattle (
33). It is also possible that the rapid placenta growth for the developing fetus occuring at this stage may account for the peak level.
There was a gradual decline in PSPB from week 10 to 15 of gestation in pregnant Yankasa ewes similar to the report of Rovani et al. (
29) in Corriedale and Ile de France cross ewes. It is also similar to the reports of Ledezma-Torres et al. (
26) in Blackheaded German Mutton, Rhoen and Dopper ewes, and the report of Batalha et al. (
22) in Alpine goats and Shahin et al. (
21) in Boer goats. However, there was variation in the pattern in Churra and Merino ewes (
18), and Assaf sheep (
20). These variations may be ascribed to differences in PSPB cross-reactivity in various breeds. Wallace et al. (
34) had attributed the variation in PSPB pattern to cross-reactivity between species and breeds. In the present study, the PSPB of pregnant Yankasa ewes gradually increased from week 15 post-breeding to the parturition (week 21.5), although there were fluctuations at weeks 18 and 20. This is similar to previous reports in sheep (
18,
20) but differs from the reports in goats (
21,
22). An increase in PSPB before parturition has also been reported in cattle (
27) and buffalo (
23). The differences may be species-specific, probably due to larger placenta mass and blood distribution through the compartments in ewe and cow. Sousa et al. (
10) highlighted these differences as possible factors that could influence PSPB concentration.
During the postpartum period, there was a steady decrease in the PSPB profile of Yankasa ewes starting from the parturition until the fourth-week post-lambing. However, PSPB was significantly different between pregnant and non-pregnant ewes up to three weeks postpartum. This is consistent with previous reports in Suffolk and Panama ewes (
25), Corriedale and Ile de France cross ewes (
29) and Boer goats (
21). However, there were variations with earlier reports in Churra, Merino (
18), Assaf ewes (
20) and Alpine goats (
22) where detectable levels were present until four weeks postpartum. In this study, basal levels, though insignificant, were also present until the fourth week postpartum. This finding supports earlier studies that reported a faster declining rate (by 28 days) in ewes compared to cows (by 100 days) (
10). The shorter half-life in sheep (4.5 days) might explain this finding (
35). The practical implication of determining PSPB levels in the postpartum period in bred Yankasa ewes would be decreasing of the false-positive results in pregnancy diagnosis.
CONCLUSION
The study showed that serum PSPB concentrations increased in pregnant ewes from week 3 post-breeding. It also showed that peak levels of PSPB in pregnant Yankasa sheep occurs at week 5, 10, and 21 post-breeding in the first, second, and third trimesters, respectively. PSPB decreased gradually after lambing until fourthweek postpartum.
CONFLICT OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTS
The authors appreciate Prof. Jerome Jefu former Executive Director and Prof. P.I. Rekwot former Assistant Director (Research) both of the National Animal Production Research Institute (NAPRI), ABU, Zaria, Nigeria for providing some of the animals used for this study. The support we received from Dr. Josh Branen of Biotracking Inc. Moscow, USA is also appreciated. We thank Mr. S. I. B. Bolaji of the Reproduction Laboratory, NAPRI-ABU, Zaria, Nigeria for his technical assistance.
AUTHORS’ CONTRIBUTIONS
AAA made the conceptualization, designed the methodology and wrote the original draft. YUA designed the methodology and was responsible for the resources. OOL was involved in the methodology design, resources and manuscript editing. IUA did the conceptualization, supervision and manuscript editing. JS was responsible for methodology design, manuscript writing and editing. KAR performed the data analysis and manuscript writing and editing. SAU was responsible for resources and manuscript editing.

 10.2478/macvetrev-2021-0010
10.2478/macvetrev-2021-0010

