Staphylococcus aureus is one of the most important causative agents of food poisoning worldwide (
1). It is an important pathogen due to its combination of toxin-related virulence, invasiveness and antibiotic resistance. One of its pathogenic properties is the ability to produce one or more staphylococcal enterotoxins (SEs), which are associated with the occurrence of staphylococcal food poisoning (SFP).
Staphylococcal poisoning is one of the most common forms of foodborne illness and occurs by ingestion of food that contains pepsin-resistant SEs produced by enterotoxigenic strains of coagulasepositive staphylococci (CPS), mainly
S. aureus. According to EFSA reports, staphylococcal intoxications are responsible for 6.4% of all foodborne illnesses and are among the top four causes of food poisoning after
Salmonella spp. foodborne viruses and
Campylobacter spp. (
2). According to the same report, staphylococcal intoxications cause a higher mortality rate (7%) compared to foodborne viruses (2.2%), and
Campylobacter spp. (1.1%). Recently, SFP has been reported to have a low hospitalization rate of 6.4%, due to the nature of the toxins and the rarely severe symptoms, usually being underreported (
3).
To this date, twenty-one SEs or enterotoxinlike proteins have been reported (
4). They are low molecular weight proteins that are classified into five main types according to their antigenic properties SEA, SEB, SEC (SEC1, SEC2, SEC3 and SEC ovine and bovine variants), SED and SEE (
4, 5), which are thought to be responsible for 95% of all staphylococcal intoxications (
6, 7, 8). The “new types” of SEs reported in recent years are designated by the initial letters from SEG to SEV (
4). SEA, which is toxic at low concentrations (0.6 ng/ml) (
9), is most commonly detected in food and is also considered to be the major cause of SFP in several countries, probably because of its exceptionally high resistance to proteolytic enzymes (75% of outbreaks), followed by SEB, SEC (mostly associated with animal origin) SED and SEE (
10).
Staphylococci in food may originate from raw materials of animal origin (e.g., milk from mastitis cows), but also from endemic strains present in the production process that may be a potential source of contamination (
11, 12). Foods of animal origin, particularly milk and dairy products, are most commonly associated with this foodborne disease. Milk is known to be an ideal growth medium for
S. aureus and its enterotoxin production, being one of the most common causes of bovine mastitis, especially subclinical mastitis (
9). During the cheese production phases,
S. aureus counts could exceed the expected count, therefore posing potential threat for SPF. Taking into consideration that approximately 10% of the cheese produced in Europe is from raw milk, good hygiene practices must always be maintained in production and processing facilities due to the potential risk for public health (
3, 13). The produced SEs retain its potency for SFP even if the
S. aureus cells are inactivated e.g. pasteurization (
3).
Many strains of
S. aureus can produce one or more enterotoxins, and the severity of the disease depends on the amount of ingested food, the SE concentration in food, and the health status of the consumer. SFP can be caused by as little as 20- 100 ng of enterotoxin. After ingestion, symptoms (copious vomiting, diarrhea, abdominal pain, or nausea) occur rapidly and abruptly (
4).
S. aureus is frequently present in the normal human microbiome (nose and hands 30-50%) and can be easily transmitted to food and working surfaces by food handling personnel (
14). Jablonski and Bohach (
15) reported that 103 to 105 CFU/g of enterotoxigenic
S. aureus strains can produce sufficient amount of SE that would pose a health risk to consumers and cause SFP.
Several tests and methods are available to determine the ability of strains to produce SEs. In addition to traditional bacteriological methods, serological tests (reversed passive latex agglutination - RPLA) and immunoassays (enzymelinked fluorescence test - ELFA) are used. These methods require an average of 3-26 hours and are capable of detecting toxin levels in the range of 0.25-1 ng/ml (
9).
Therefore, the study aimed to obtain data on the prevalence and counts of
S. aureus strains in raw milk and cheese, and to determine the number of strains capable of producing enterotoxins by using the SET RPLA detection method by passive agglutination and VIDAS SET 2 which is an enzyme-linked fluorescence test (ELFA).
MATERIAL AND METHODS
SamplingThe raw cow milk and cheese samples were obtained from fourteen different dairies in North Macedonia between October 2017 and January 2018. One hundred and fifty-three samples of raw cow’s milk were collected immediately after arrival in the dairies from various milk producers and were transported to the microbiology laboratory in 60-ml sterile cups at 4-8 ºC within 4 hours.
Seventy-five samples of different cheese types (cow’s and sheep’s) and twenty samples of yellow cheese, aged between 2 weeks and 2 months were collected. The cheese samples were produced from cooked milk and were collected on several occasions, at different ripening periods. The sampling was performed from commercial packaging and the storage and transportation conditions were the same as for the raw milk samples.
Isolation and enumeration of S. aureusSamples were analyzed for enumeration of coagulase-positive staphylococci (
S. aureus) using TEMPO STA cards and then strains were confirmed according to ISO 6888-1 “Horizontal method for enumeration of coagulase-positive staphylococci” (
16), with additional identification using GP cards of the VITEK 2 automatic identification system (Biomerieux).
The TEMPO system (bioMérieux, Marcyl’Étoile, France) is the first semi-automatic system detecting the number and volume of positive wells (fluorescent or non-fluorescent) in the specific test card and enumerating bacteria by utilizing statistical methods based on the most probable number (MPN). It is AFNOR certified and AOAC validated. The TEMPO system is increasingly used in the food industry due to its semi-automated, traceable, and fast process for bacteriological counting and identification without the need for additional identification tests. We used the TEMPO STA test card for each raw milk sample. The suspension of the medium containing 1 ml of the sample was distributed in the 48 analysis wells of the test card with three different volumes (225, 22.5 and 2.25 μl), from which a fluorescence signal can be recorded and interpreted to derive the initial bacterial content, expressed in CFU/ml. The TEMPO STA test card was used to determine the
S. aureus concentration after incubation at 35 °C for 24-27 h. After each incubation period, all cards were analyzed using the TEMPO reading station and the results were expressed in CFU/ml for the original sample after manual entry of the initial dilution factor. Taking into account that we used 1 ml of raw milk in the initial suspension, we were able to detect
S. aureus counts ranging from 1 to 4900 CFU/ml.
The evaluation of the acceptability of raw milk regarding the presence of
S. aureus in raw milk was carried out in accordance with Article 19 of the Book of rules on the specific requirements for safety and hygiene and the manner and procedure of carrying out official controls on milk and milk products (Official Gazette of RM no. 26/2012). Acceptable raw milk samples were considered if 2 out of 5 tested units would have
S. aureus count between 500 and 2000 CFU/ml. If only one unit exceeded count of 2000 CFU/ml (
17), the raw milk sample was considered unacceptable.
According to the Book of rules for microbiological criteria of food safety Off. G. of R. M. no100/2013, we took 5 units per cheese sample to see if the criteria for enumeration of coagulasepositive staphylococci were met. Samples with a count above 1000 CFU/g were considered unacceptable (
18).
Detection of the SEsStrains were obtained from the following sources: raw cow’s milk (n=108), various cheeses (n=17), and hard yellow cheese (n=5). One colony per sample was tested. We investigated the ability of
S. aureus strains to produce enterotoxins using the reverse passive latex agglutination test SET RPLA detection kit (includes detection of SEA, SEB, SEC and SED) (Oxoid, UK). According to the manufacturer, the sensitivity of the test was 0.5 ng/ml of the tested extract, i.e. bacterial suspension. All
S. aureus isolates were incubated in Tryptone soy broth (CM0129B, Oxoid-UK) for 12-18 h at 37 ºC. Subsequently, all broth cultures were filtered and the filtrate was used for the assay by reverse passive latex agglutination SET-RPLA (TD0900A, Oxoid-UK) according to the manufacturer’s instructions.
The second test (VIDAS SET 2) was used to detect the ability of strains to produce SEs (BioMérieux SA - France). The test is an enzyme-linked fluorescence assay (ELFA) used for qualitative and semi-qualitative detection of 7 types of enterotoxins (SEA, SEB, SEC1, SEC2, SEC3, SED and SEE). The manufacturer’s general protocol was modified with SET RPLA and ENTEROTOX-F (Denka Seiken Co., Ltd., Japan) extraction. The supernatant was further processed according to the manufacturer’s instructions (
19, 20).
RESULTS
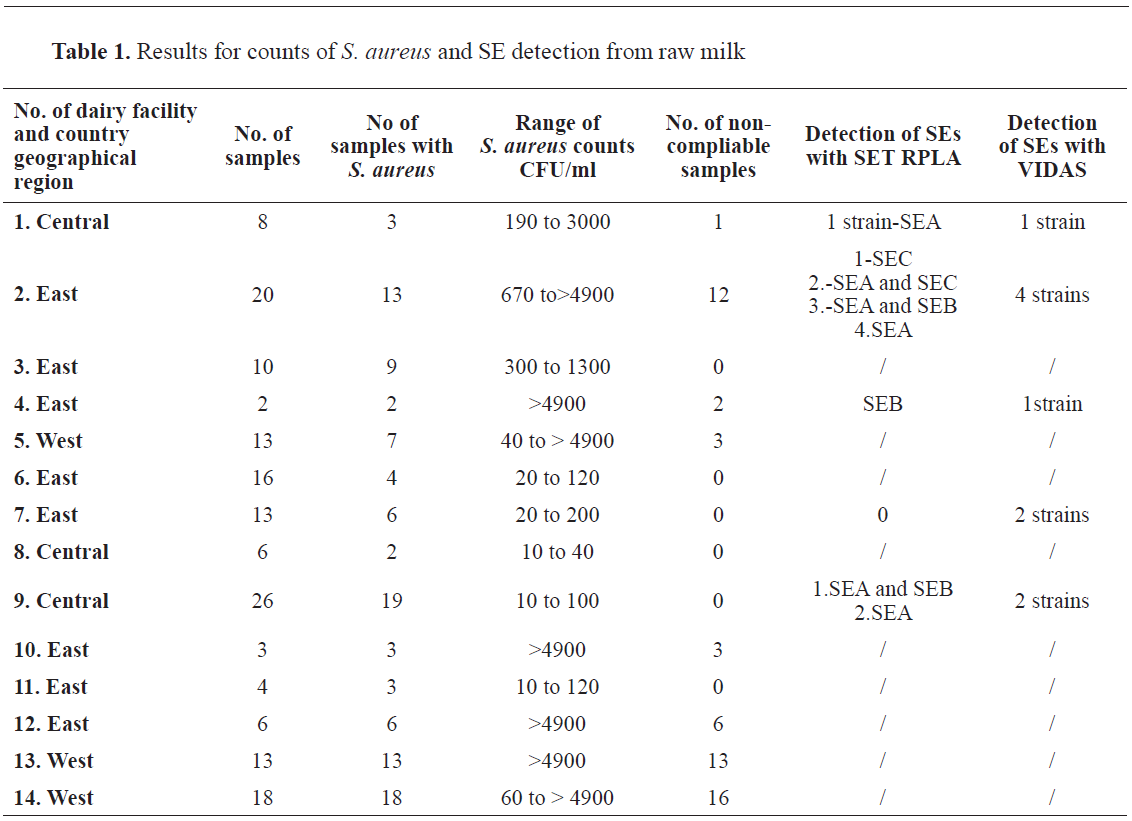
In 153 raw cow milk samples,
S. aureus was detected in 108 samples (70.6%). The number of colonies per sample varied in a wide range (
Table 1). In 56 samples (36.6%) the bacterial count was higher than 2000 CFU/ml deeming them unacceptable according to the described criteria (
17). The ability of the strains to produce SEs is shown in
Table 1. A total of 10 (6.5%) strains were positive by VIDAS SET 2 method, and 8 (5.2%) by SET RPLA method (SEA: n=3, SEB: n=1, SEA and SEB: n=2, SEA and SEC: n=1, SEC: n=1).
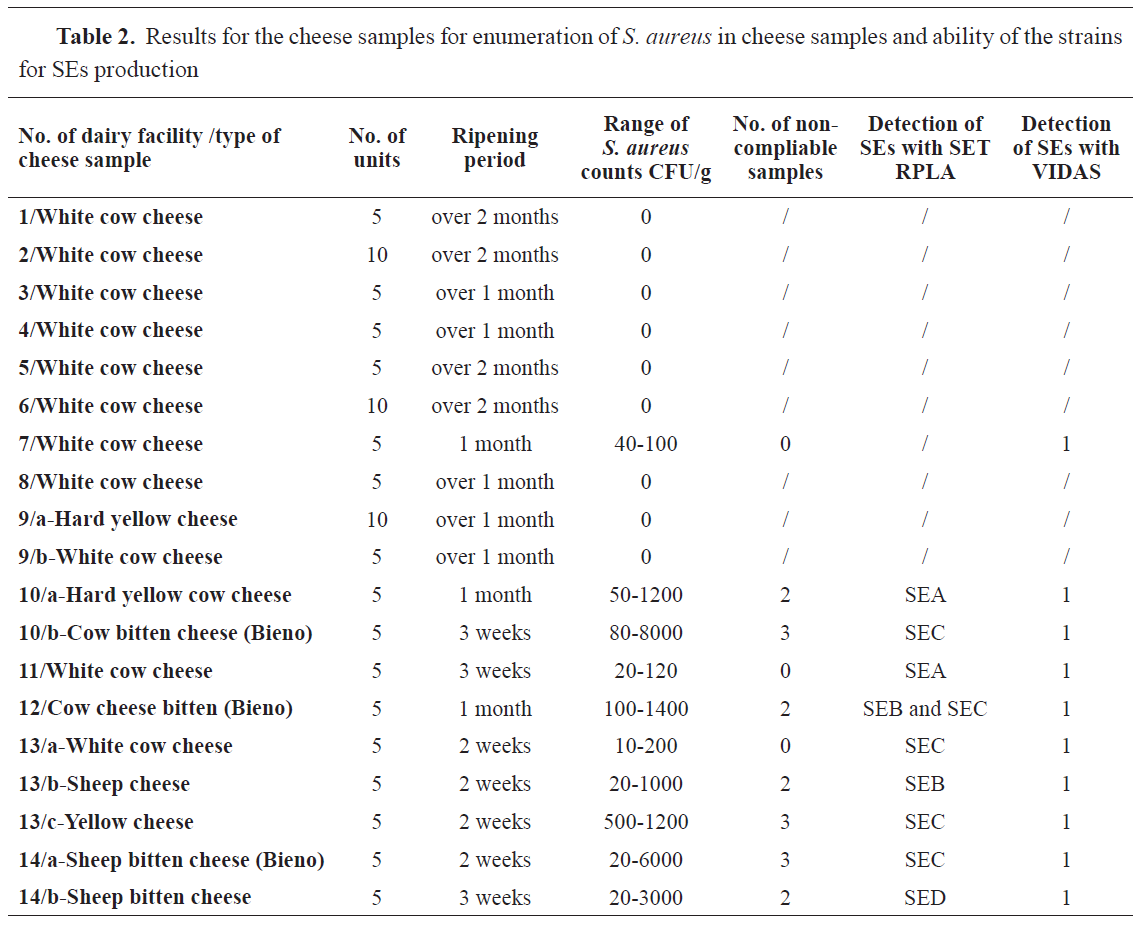
From 75 samples of different types of cheese and 20 samples of hard yellow cheese
S. aureus was found in 22 (23.1%) samples. Twelve units from all cheese samples (16%) and five units (25%) of the hard yellow cheese samples were unsatisfactory according to the previously described criteria (
18).
The ability of
S. aureus strains obtained from cheese samples to produce SEs was confirmed in 10 (10.5%) by VIDAS SET 2 and in 9 (9.4%) by SET RPLA kit. The ability to produce SEC was detected in 4 strains (44.4%), SEA in 2 (22.2), SEB in 1 and SEB with SEC in 1 strain. The data for the cheese samples are shown in
Table 2.
DISCUSSION
The incidence of staphylococcal intoxication is usually underestimated or unreported due to undetectable and mild symptoms leading to misdiagnosis, unreported minor outbreaks, improper specimen collection and improper laboratory testing.
The prevalence of enterotoxicity is reported differently by various authors and countries due to the diversity of bacterial strains and their characteristics. Our findings are in agreement with the report of Ertas et al. (
21) for
S. aureus counts and with the report of Dzirba - Korpysa W. and Osek J. (
22) for the prevalence of enterotoxic strains
S. aureus.
All samples in the current study had a lower
S. aureus count than the specified limit (>105 CFU/g in a product) according to Article 1.28 in the previously stated Book of rules (
18). They would not be routinely subjected to testing for SEs presence. Nevertheless, some samples of retail cheese had sufficient bacterial counts that under appropriate environmental conditions, could produce sufficient SEs concentration leading to SFP (
23).
Despite the wide discrepancy in data on the prevalence of enterotoxigenic
S. aureus isolates in the literature, studies conducted in Germany, Brazil, Japan and the United States found a prevalence of enterotoxin genes in
S. aureus isolates ranging from 10% to 70% (
9). This variability may be due to differences in sampling (severity of clinical manifestation – mastitis), geographic location, laboratory methods and variations in enterotoxin identification methodology. SEC and SEA are most commonly detected in isolates from cow and human samples, respectively. In the current study, 8 strains with SEA enterotoxicity (47% of SET RPLA-positive samples), 7 SEC (41.1%), 5 SEB (29.4%), and 1 SED strains (5.8%) were isolated. Four strains (23.5%) were able to produce 2 types of SEs (two strains with SEA and SEB, SEA and SEC, SEB and SEC).
In 3 (2.3%) raw cow milk and cheese samples originating from the same dairy, the SET RPLA (SEA to SED) and the VIDAS SET 2 (SEA to SEE) reports were different. This discrepancy could be explained due to the low SE concentration, under the detection limit of the SET RPLA test, or due to the possible presence of SEE strains undetectable by the SET RPLA. Nevertheless, both methods were highly correlated in detecting SEs (
9).
CONCLUSION
The results of our study showed that the prevalence of enterotoxigenic strains of
S. aureus obtained from different matrices. These results highlight the importance of proper sanitation and hygiene practices, the use of safe raw materials and proper handling of finished products, and the need for further monitoring of the situation with SEs to have safer food and avoid SFP outbreaks. Further efforts should be made in developing methods (PCR) and detection kits (VIDAS technology) that could detect a broader spectrum of SE.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
This research was supported by the Faculty of Veterinary Medicine – Skopje, and the authors would like to thank colleagues from the Department of Microbiology of food and feed at the Food Institute for the cooperation and contribution of this study.
AUTHORS’ CONTRIBUTION
MRM conceived the study, did the experimental examinations and wrote the мanuscript. LjA contributed with the collecting of the raw milk samples. MP, DJ and PS contributed to the study design. PS supervised the study, gave critical revision and contributed to the final version of the manuscript.

 10.2478/macvetrev-2021-0014
10.2478/macvetrev-2021-0014

