Due to the unique morphological, anatomical, and physiological features of the urinary bladder (UB), it is used for bio-modeling of various pathological processes (
1, 2, 3, 4, 5). The experimental studies on the UB of the laboratory animals help to understand the pathogenesis of various diseases which can be used for extrapolation of the results to human medicine (
1, 2, 3, 6, 7, 8). The disruption of the mucous membrane integrity often correlates to the development of various pathologies and urination disorders (
1, 2, 4, 7, 8, 9, 10). The urothelium surface is normally resilient to the biochemical components of the urine, microorganisms, and toxins. The local protection of the mucosal cells includes the tight junctions of umbrella cells (physical barrier) (
5, 9), glycosaminoglycans (
9, 11), cytokeratin (
12), uroplakins (Upk1a, Upk1b, Upk2, Upk3a and Upk3b), and numerous membrane receptors (
9, 13).
Few nematode species can parasitize in animals’ UB (
14, 15). Depending on the development stage, they can migrate to nearly all organs and systems with or without clinical manifestations (
15). They may affect the tissue structure and normal physiological processes disrupting the micturition.
Trichosomoides crassicauda (
T. crassicauda) is a nematode commonly localized in the UB of laboratory and wild rats (
14, 15, 16, 17). It was also detected in the kidneys, uterus, and lungs (
17, 18). The nematode eggs are released in the urine (the eggs remain viable for a long time at room temperature) and enter another host’s digestive system by contaminated food (water and food products). They are hatched in the stomach and reach the kidneys and UB by blood circulation. The helminths are accumulating in the urine and UB cavity where they lay the eggs in the mucosal tissue at a very early stage of their development. The eggs continue to develop repeating the same cycle of events. The prepatent period including the time between infection and the production of eggs is 8-10 weeks (
15, 16, 17, 18). They can also be transmitted from the mother to the offspring before weaning (
16). It is important to note that the biological behavior and pairing of the heterogeneous
T. crassicauda are unique due to the different body sizes in males and females. Female nematodes are larger than males which is significant for the evolution of sexual selection. The smaller male nematode penetrates to the genital tract of the larger female nematodes fertilizing the eggs. Females are more frequently localized in the UB, while the males in the uterus (
15, 17, 18, 19).
The absence of these nematodes in the urine does not exclude their presence in UB. Therefore, for positive determination of this parasite, it is suggested that both urine and UB tissue must be checked (
15). The mechanisms of
T. crassicauda parasitism have not been studied sufficiently. A positive correlation was described between pathomorphological changes in UB and uterus (inflammation and tumors) (
15, 17, 18, 20). This confirms that the presence of
T. crassicauda may negatively impact on the results of a modeling study. Therefore, this research aims to comprehensively study the features of the morphological changes in the UB wall of white laboratory rats due to the presence of
T. crassicauda.
MATERIAL AND METHODS
Animals and experimental design In this bio-modeling study, 60 male albino laboratory rats (214.62±12.40 g) were acquired from the Medical Institute of Sumy State University, Animal House. Animals were allowed to acclimatize for one week before the experiment had been commenced. During the study, the rodents were housed in polypropylene cages. They were kept in a room under a controlled constant temperature of 24 ºC±1 ºC, relative humidity of 55%±5%, 12 hours light/dark cycle, free access to tap water, and standard laboratory animal food. Urine samples were collected in three consecutive days of the experiment, at the same time (from 10 am to 1 pm). They were microscopically analyzed for the presence of the parasite (magnification ×100). According to the findings, the rodents were divided into two groups – presence (group 1 - infected group) and absence of
T. crassicauda (group 2 - control group).
They were anesthetized and euthanized by cervical dislocation. The UB was identified and excised by a midline abdominal incision. Animal handling was performed with consideration to minimize stress to the experimental animals. All experiments were performed according to the guidelines established by the European Community for the Care and Use of Laboratory Animals, Ethics and Animal Welfare Committee of the International Council for Laboratory Animal Science and according to local Ethics guidelines, and were approved by the Institute Bioethics Commission (experimental protocol № 25/04 from 10.04.2018).
Tissue preparation, morphometric scoring, histopathologic and immunohistochemical examination
After excising UB tissue from the animals, samples were immediately fixed in 10% formalin solution for 24 h, dehydrated (in ascending grades of ethanol 70–96%), cleared in xylene, and embedded in paraffin (4% wax). Thereafter, each block was cut into 4 μm thick sections on rotational microtome Shandon Finesse 325 (Thermo Scientific, USA), placed on aminopropyltriethoxysilane-coated slide glasses (Thermo Scientific, USA), deparaffinized, and rehydrated (xylene and descending graded ethanol 96–70%) by routine methods. Tissue samples were stained with Hematoxylin-Eosin and Alcian Blue techniques and mounted by Canada balsam (Thermo Scientific, USA) according to the standard method (
21). Urine specimens were diluted with 10% formalin and examined under a microscope on slide glasses. Helminth species and eggs were confirmed by shape, size, membrane thickness and color (
19).
Other samples were stained immunohistochemically. Antigen retrieval was carried out in a water bath at 97-98 °C containing boiled citrate antigen retrieval buffer, pH 6.0 (Thermo Fisher Scientific, USA) for 30 min. The cooled slides were washed in distilled water and placed in Tris-buffered saline (Thermo Fisher Scientific, USA) pH 7.4 for 20 min. The antigenantibody reaction was visualized using the Ultra Vision Quanto Detection System HRP DAB Chromogen (Thermo Fisher Scientific, USA), which included blocking endogenous peroxidase activity by 3% H2O2, protein blocker using the Ultra V block and enhancing the reaction by Primary Antibody Amplifier Quant. Primary antibodies in different dilutions were used for determining the expression of receptors to Ki-67, Hsp70, Hsp90α, CD3 and CD20. Immunohistochemical analysis was visualized using the polymer detection system with diaminobenzidine chromogen, and additional nuclei counterstaining with Modified Mayer’s Hematoxylin (Thermo Fisher Scientific, USA), dehydrated, cleared and mounted. All morphological examinations and morphometric scoring of parasites and their eggs were performed using «Carl Zeiss Primo Star» microscope with a digital camera «Zeiss AxioCam ERс 5s» and the software package for the image output «ZEN 2 (blue edition)» (Germany) with a built-in micrometer and calculation output.
Statistical analysisThe results were presented as Mean ± SD or as a percentage of variation compared to the control.
RESULTS
The urine analysis revealed the presence of
T. crassicauda eggs in 17 (28.33%) rats (group 1) with significant variation in the number in each specimen. Group 2 included rats without nematode infection in urine – 43 (71.67%). Morphological analysis of UB confirmed changes in both groups: animals in group 1 (17 rats – 100%) and group 2 (8 rats – 18.6%). The overall rate of infection was 41.67 % (from the total number of animals - 60 rats).
Helminths and their eggs were localized in the UB cavity (in the lumen and between mucous rugae) and/or embedded in the epithelium.
T. crassicauda had an elongated body with rounded ends, mature and/or immature eggs. Morphometric characteristics of the females of
T. crassicauda significantly varied and in average were 612.28±153.71 μm in length and 94.13±19.71 μm in width. The male specimens outside the female body were found only twice in the lumen of the UB (first one – 47.15 μm in length and 8.20 μm in diameter, the second one – 38.26 μm in length and 7.90 μm in width).
On histological examination, we found a discrete swelling in the UB, disturbed stratification of the urothelium, and localized hyperplasia. The helminths and eggs were encapsulated by transitional epithelial cells. The adjacent cells had dystrophic and necrotic changes. Desquamations caused localized increased permeability of the mucosal layer. The helminths were localized on the top or between the rugae without inflammatory reaction in lamina propria and submucosa. Despite the direct contact of the nematode eggs with lamina propria, single immune cells were observed only in several rats (
Fig. 1).
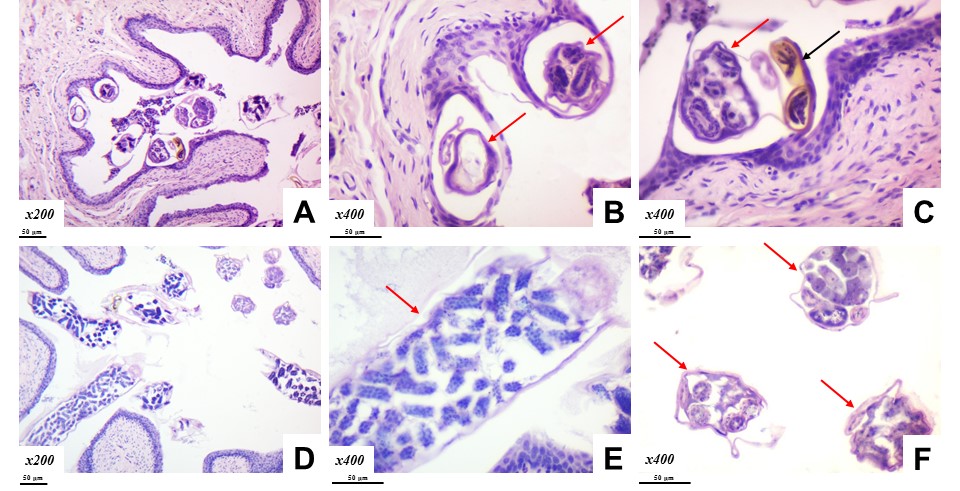 Figure 1. Trichosomoides crassicauda
Figure 1. Trichosomoides crassicauda (red arrow) and eggs (black arrow) in the rat UB. Hematoxyline and Eosin staining. Magnification: A, D×200; B, C, E, F×400. Scale bar=50 μm
Despite the varying female size, the eggs did not have significant variations (59.14±6.69 μm in length and 37.76±7.89 μm in width) which were influenced mainly on the development stage of the female and its eggs.
T. crassicauda`s eggs had a well-defined brown oval capsule (3.26±0.71 μm thick) the embryos being tightly situated in its cavities. Nematodes and their eggs penetrated different layers of the urothelium, mostly reaching the basement membrane of UB by passing through the umbrella, intermediate, and basal cells where they formed chamber-like capsule around them. Although they were embedded in the transitional epithelium, a small space was visible around their shell. The chamber-like capsule encompassed single or multiple parasites in its cavity.
The glycosaminoglycans layer of the urothelium was not entirely eroded despite the disrupted stratification of the cells. The affected layers included the epithelium and the surrounding encapsulated parasite areas (
Fig. 2 A, B, C).
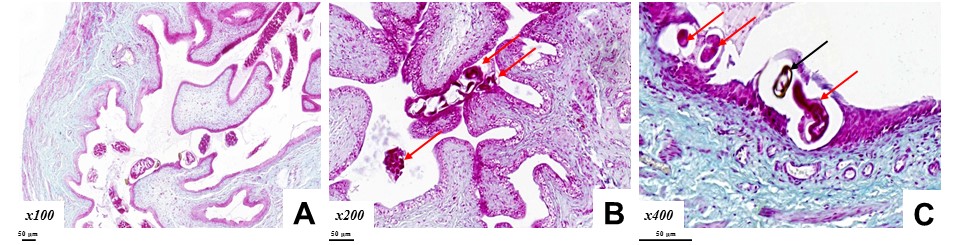 Figure 2. Trichosomoides crassicauda
Figure 2. Trichosomoides crassicauda (red arrow) and eggs (black arrow) in the rat UB. Alcian Blue staining. Magnification: A×100; B×200; C×400. Scale bar=50 μm
A low proliferative index of the transitional epithelium was observed throughout the mucous membrane. Ki-67 positive expression was found in single cells of the basal and partly intermediate layers of the transitional epithelium. Ki-67 was mainly not expressed by cells in the affected and surrounding areas or its expression was sporadic in few cells (
Fig. 3 A). Increased proliferative activity was found near the desquamation lesions of the epithelium.
The surrounding epithelial tissue showed an increase of the chaperones’ activity around the parasite localization. Expressed induction of Hsp70 and Hsp90α was observed in basal, intermediate and umbrella cells (
Fig. 3 B, C).
An immunohistochemical study confirmed the absence of inflammatory infiltration. CD3 (T-lymphocytes) and CD20 (B-lymphocytes) were detected in small and solitary formations around the helminths and eggs in the UB mucous membrane (
Fig. 3 D, E).
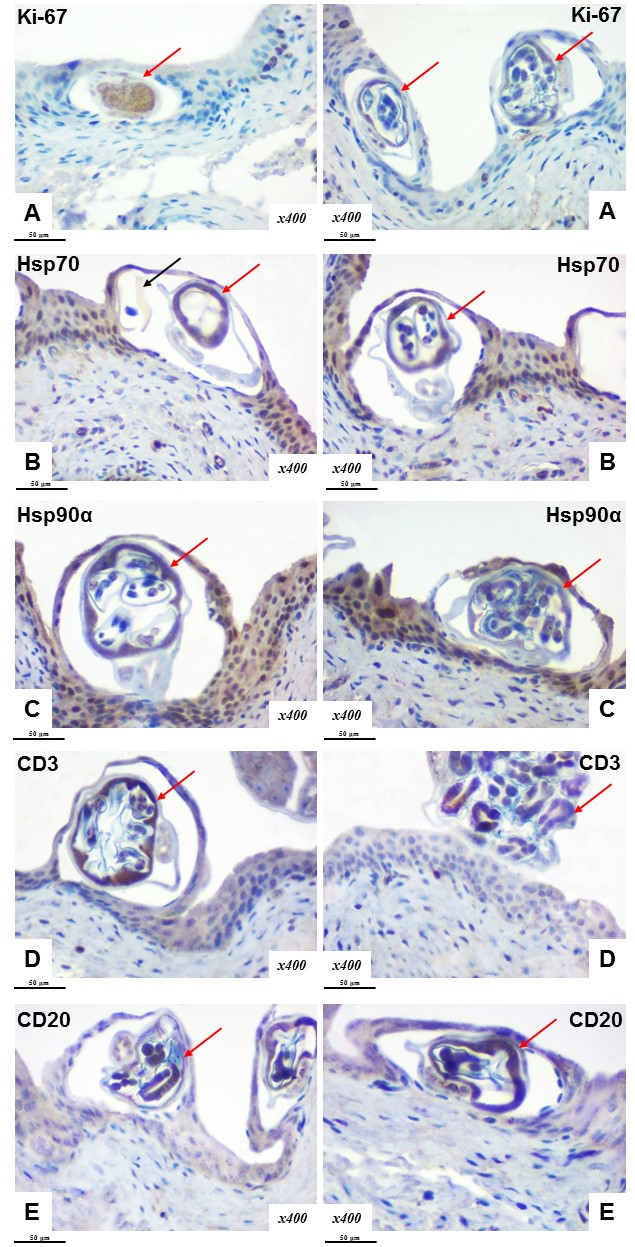 Figure 3.
Figure 3. Intraepithelial localization of
Trichosomoides crassicauda (red arrow) and eggs (black arrow) in the rat UB. Immunohistochemical examination of Ki-67 (A), Hsp70 (B), Hsp90α (C), CD3 (D) and CD20 (E) expression. Chromogen – diaminobenzidine. Nuclei were counterstained with Mayer’s hematoxylin. Magnification: ×400. Scale bar=50 μm
DISCUSSION
Rats are frequently used as experimental animals for pathological and/or biomodelling studies which are essential for improving theoretical understanding (
22). It is generally believed that numerous threats with unclear pathogenesis can affect UB functioning and lead to urodynamic disorders (
2, 3, 5, 9, 13). The causes of tissue transformation under the influence of exoand endogenous factors are a topic of continuous investigations (
3, 9, 23).
Parasites are considered as one of the most frequently observed macroscopic pathological agents (
19, 24, 25). In our study, we investigated the influence of
T. crassicauda on UB. The presence of these nematodes in the UB is correlated with the development of cystitis, bladder stones, metaplasia, and oncological processes (
14, 17, 18, 19, 20). Moreover, they were revealed both in wild and laboratory rats despite the significant habitual differences. However, its’ effects on UB are not sufficiently elucidated and is still an ongoing topic in current research (
15, 16, 17, 18, 20, 26).
In urine samples,
T. crassicauda was detected in 28.33%. However, by adding the findings of the morphological identification in UB samples, this value was increased by 18.60%. This indicates that urine analysis alone cannot be used for the detection of the presence of this parasite in the UB (15, 16). It should be noted that despite the experimental conditions which allowed close contact between the animals, the possibility for cross-infection was significantly low. In natural and experimental conditions, the infestation level has a certain value in the population despite the high zoonotic potentials of these parasites. Based on the results obtained by Mustapha Tijjani et al. (
27), intestinal or tissue parasites were found in 17.1% and 15.4% of wild rodents, respectively. Some of the rodents were infected with various species of parasites.
It is considered that mature males and females differ in susceptibility to infections caused by bacteria, fungi, parasites, and viruses due to their innate physiological sex differences, genetic and behavioral features, endocrine-immune interactions, hormones profile, and their effects on gene expression. (
28). All these gender differences may explain some variability in response to various infections. This might be explained due to the specific immune function affected by circulating steroid hormones (testosterone, estradiol, progesterone, and glucocorticoids) (
29). Accordingly, men are more susceptible to parasitic infections due to the immunosuppressing role of testosterone (
15, 28, 19, 30). Although the prevalence and intensity of infections are higher in males than in females, there are also parasites for which males are more resistant than females (
28, 29).
This research has demonstrated the effects of direct contact interaction between the parasite and the UB epithelium. Changes in the transitional epithelium had a pronounced heterogenicity due to the increased susceptibility for secondary infections. This can be found in other reports where urothelium’s responsiveness is described with granulocyte infiltration) and hyperplasia resulting in variable outcome (
15, 16, 17, 18, 20). We observed solitary inflammatory cells without parenchymalstromal components in the UB.
The UB responsiveness to infectious/parasitic insults is mainly dependent on mucosal integrity (
3, 9). The helminthiasis of UB is mainly characterized by disruption of the glycosaminoglycans layer with a consequent increased permeability. The parasite is usually localized beneath and between the mucosal cells of the UB. We observed that the alterations of the mucosal cells surrounding the encapsulated parasite can be partially restored with permanent deterioration of the superficial layer. It is presumed that the location of the parasite in the deeper mucosal layers may be to avoid direct contact with the urine at certain stages of their development. This results in recurrent urinary tract infections, interstitial cystitis, urolithiasis, and neoplastic changes (
1, 3, 10, 11, 13, 20, 25).
The tissue inflammatory response (number and localization of immune cells) may indicate the outcome of the pathological process caused by various exo- and endogenous factors. (
2, 3, 4, 9, 23, 31, 32, 33, 34, 35). During our study, only single T/B-lymphocytes were observed in the mucosa. Prolonged contact of
T. crassicauda with UB mucosa may cause inflammatory response with variable degrees of pathomorphological changes depending on other factors. The insignificant changes and relatively low immune response may be related to the high regenerative capabilities of the UB mucosa (
36).
The presence of Ki-67 in few cells was not entirely elucidated. Ki-67 was detected in areas with epithelial lesions (desquamation areas), but it was almost undetectable in mucosal cells surrounding the encapsulated parasite. It is considered an essential element of regeneration (
2, 20, 37). The deteriorated transitional epithelium structure and integrity were not accompanied by processes of proliferation which may be explained due to the lack (blocked) of cell receptors.
Heat shock proteins (Нsp70, Нsp90α) act as protective components of the epithelium and are actively involved in their local defense (
38). The cytoprotective properties of chaperones in the epithelium have been reported following a few hours or days after tissue insult (
2, 39). The long-term impact of different factors on UB and chaperones’ reactions remains unclear. Increased expression of Нsp70 and Нsp90α in urothelium around helminths probably appeared as a response to cellular stress.
It is a compensatory reaction of normal cells to recover proteins’ conformation, preventing caspases activation and apoptosis. Similar data on the full range of their protective properties are described in other reports (
2, 38, 39, 40).
In summary, from the UB lumen, the parasite is breaching the glycosaminoglycans layer penetrating the epithelial physical barrier and continues its development in the mucosal layers. The variable pathomorphological and pathophysiological changes in the UB mucosa may lead to uncertain predictions of the parasite’s pathological patterns. Additional research would elucidate these mechanisms.
CONCLUSION
The nematodes
Trichosomoides crassicauda and their eggs are frequently found in the urinary bladder of laboratory rats. Their absence in urine does not exclude the helminthiasis in the urinary bladder. The co-existence of rats (healthy and infected with parasites) does not necessarily lead to cross-infection to all members of the population.
The nematodes disrupt the stratified structure of the urothelial cells bypassing the glycosaminoglycans’ protective layer and encapsulating in the mucosal layers. This process is accompanied by a small-scale inflammatory reactions and other pathological changes. The lack of increased proliferative activity and increased chaperone expression may indicate to natural defense for this parasite.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENT
This research was supported by the research themes № 0119U100887 and № 0121U100472.
AUTHORS’ CONTRIBUTION
ML and VS were involved in literature review, design, preparing the manuscript and figures. AR, RM, ML and VS were responsible for final approval of the article, analysis and data interpretation. NH, YL, KS, RC and OD conducted the experimental part, data acquisition, statistical analysis, participated in writing, editing and discussion.

 10.2478/macvetrev-2021-0019
10.2478/macvetrev-2021-0019


