Due to the actuality of spongiform encephalopathies and their proven spreading by means of animal feed containing meat and bone meal, the description and measurement of osteocytic lacunae contributes to more easily distinguish bone fragments in meat and bone meal. Transmissible spongiform encephalopathies (TSEs) have attracted a lot of attention, especially after 1986, when the first case of BSE (bovine spongiform encephalopathy) was detected. Since the outbreak of spongiform encephalopathy (BSE), the use of animal protein including bone meal as an ingredient in animal feed has been controlled by several regulations including Regulation (EC) 999/2001, Regulation (EC) 1774/2002, and Regulation (EC) 1234/2003. The classical microscopic method is the only official method for detecting animal protein in animal feed in the European Union (Commission Regulation (EC) 152/2009). By applying the microscopic method to the animal feed samples, we performed detection in order to determine the presence of animal proteins that originate from mammals and fish. The microscopic analysis of all 421 samples, of which 115 were raw materials for the production of animal feed, 230 were concentrates for ruminant nutrition and 76 were concentrates for non-ruminant nutrition (32 concentrates for laying hens and 44 concentrates for pigs), did not provide positive results, that is, no remains of animal tissues of mammalian origin were found in any specimen. Whereas in 10 out of 32 (31.25%) concentrates intended for non-ruminant nutrition (laying hens), pieces of fish tissue were found. In these samples, we usually detected the presence of fish bones, gills and scales.
After the discovery of the group of prion diseases in farm and domestic animals, which belong to the group of transmissible spongiform encephalopathies (TSEs), since 1996, a ban to use meat and bone meal that originates from mammals in ruminant nutrition has been introduced in all countries of the European Union. Prions are infectious particles smaller than viruses, built only of proteins. Their composition does not contain nucleic acids. They were discovered by a group of researchers from the University of San Francisco (
1).
Prions are ten times smaller than the smallest virus, their size is 2-3 nm. Prions are temperature resistant. They can be inactivated at a temperature of 130°C in one hour. They are also resistant to UV radiation as they do not contain nucleic acids. Consequently, they are also resistant to enzymes that break down nucleic acids - PrPc and PrPsc nucleases are two forms or two isomeric forms of a prion. Meat and bone meal is a protein raw material produced by heat treatment of animal waste in incineration plants. Meat and bone meal, feather meal and fish meal contain a higher percentage of amino acids than soybeans, and at least five essential amino acids. The amino acid uptake from meat and bone meal is between 60-80% (
1).
The ban on the use of meat and bone meal in the nutrition of farm animals has led to major losses, and due to the introduction of plant replacement it has led to changes in agricultural policies for the cultivation of crops (
2). Therefore, the possibility to annul the ban on the use of meat and bone meal in the nutrition of farm animals is being considered.
Studies have shown that the source of contamination was food containing meat and bone meal, originating from ill animals (
3). A consumption of 1-100 mg of infected tissue is sufficient for a bovine to become ill (
4). Pigs are susceptible to this disease, only parenteral infection has been confirmed in pigs (
5). Transmissible encephalopathy has not been found in chicken and fish so far (
6). However, it is considered that prions, triggers of transmissible encephalopathies, may circulate among species that are not susceptible to the disease, but still represent potential sources of contamination in the food chain (
7, 8).
Animal feed control for the presence of meat and bone meals provides the best results when analyzing bone lacunae. The largest difference in the morphology of the lacunae was observed between the bone lacunae of fish, mammals and birds. They may look like longitudinal lines on bones without canaliculi, or have distinctly elongated, spindle-like lacunae with long canaliculi (
9). Domenis et al. (
10) describe bird lacunae as densely distributed, with hardly observable canaliculi using software (ARIES, 2004). Unlike birds, the bones of terrestrial mammal have elliptical lacunas with relatively well-visible canaliculi. Pig bones differ from this description because they are more densely distributed and resemble bird bones (
10).
According to Directorate General for Health and Consumer Protection of the European Commission (DG SANCO), and their TSE-related guidelines, it is proposed to annul the ban on feeding pigs, chicken and fish with meat and bone meal only if cannibalism is prevented (
18). In any case, re-introduction of meat and bone meal into ruminant nutrition is not foreseen. Animal feed that contains animal constituents must be with a declaration. One of the measures is to improve and train staff at food mills. The development of improved feeding practices for animals intended for food production, the improvement of food production systems and the improvement of sampling techniques and analytical techniques suitable for the analysis of animal feed also ease the problem.
Experiences from countries such as the United Kingdom have shown that strict control of animal feed ingredients helps to reduce the number of infected animals. The use of food that is high in proteins is essential when it comes to animal yield, especially lactation. By the mid-1970s, the price of plant proteins in the United States had grown to a point when it was concluded that the diet with animal proteins was increasingly popular in cattle nutrition. In Canada, meat and bone meal was rarely used for bovine nutrition in the period before 1997. For dairy cows, higher meat and bone meal content is required to achieve higher production (more than 8000 kg per cow in lactation). Their meals contain about 200 g of blood meal per day in the first 70 days of breastfeeding.
MATERIAL AND METHODS
A total of 421 samples of different animal feed were examined for the presence of animal protein. Among them, 230 samples were taken from livestock feed (concentrates for ruminant animals), 115 were from raw materials for the production of livestock feed and 76 samples were from concentrates for non-ruminant animals. The sampling process took place from May 2017 to December 2018. All examined samples were taken from different sites.
The identification of animal tissues is performed based on the microscopic identification characteristics (eg. bones, muscle fibers, horns, cartilages, blood, fibers, feathers, eggshells, fish bones). The identification was conducted on a concentrated sediment from the sample by using a binding and coloring reagent. In mixed feed, the concentrated sediment typically contains not only parts of animal and fish bones, but also particles with high specific weight such as: minerals, sand, plant parts, etc.
We performed microscopy with a microscope of the brand NIKON ECLIPSE E600, with a magnification of 4x0.10 and 10x0.25, and the sample capturing was performed with a DinoEye camera.
Reagents-Binders• Chloral hydrate (60%). The cellular structure can be seen more clearly, because the starch grains are gelatinized and the unnecessary cellular content is removed.
• 2.5% NaOH or 2.5% KOH for sieve fractions. NaOH and KOH purify the food material, helping to detect muscle fibers, fibers and other keratin structures.
• Paraffin oil or glycerol (viscosity: 68-81) for microscopic observation of the sediment.
-
Rinse agents - Alcohol 96% and Acetone
-
Concentrator - Tetrachlorethylene (density 1.62)
-
Coloring reagents• Iodine/potassium iodide solution, it is used for detection of starch (blue-violet) and protein (yelloworange).
The solutions may be diluted if necessary.
• Alizarin red. Red/pink color of bones, fish bones and scales.
• Cysteine reagent. The hairs, feathers and plant residues turn black and brown.
-
Bleaching reagents• Commercially available sodium hypochloride (9.6% active chlorine)
Bone constituents are well identified with these reagents because most “lacunae” (voids, cavities) remain filled with air and look like black holes with
a size of 5-15 μm.
ProcedureWe transferred at least 5 g (with an accuracy of 0.01 g) of the sample to a separation funnel with a conical bottom and added at least 50 ml of tetrachlorethylene. The mixture was mixed well and was repeated several times, allowing the precipitate to stand for three minutes. Then we released the sediment. The dried precipitate was examined for the presence of bone constituents under a stereomicroscope. We transferred the entire precipitate to a glass tube and rinsed it twice with about 5 ml of alcohol (each time we used vortex, we left if for one minute and then poured it). Before dyeing the precipitate, we bleached it by adding 1 ml of sodium hypochlorite, which was left to act for 10 minutes. We filled the tube with distilled water and allowed the precipitate to normalize for 2-3 minutes, and then poured the water and the suspended particles. The precipitate was rinsed two more times with 10 ml of water (we used vortex, allowed it to normalize and poured water every time). We added 2 to 10 drops (depending on the amount of residue) of the alizarin red solution. The mixture was shaken and the reaction took place within seconds. We rinsed the colored precipitate twice with approximately 5 ml of alcohol followed by one rinse with acetone (each time we used vortex, we allowed the solvent to normalize for one minute and then we poured it). In this way, the sludge was ready for drying. The fraction that remained in the funnel after the sediment was discharged was transferred quantitatively to a glass vessel where we left it to dry. We examined the dyed dry fraction with cystine reagent for the presence of fibers, feathers, and plant cells. In fodder mixtures, the concentrated sediment should contain not only bones of terrestrial animals and parts of fish bones, but also other particles of high characteristic weight, such as minerals, sand, hardened plant parts, and the like.
The percentage of bones in products of animal origin can vary greatly. The percentage of bones in case of bone meal can range from 50-60%, and in the case of bone meal it can range from 20-30%, in case of fish bone and scales meal the content may vary depending on the category and the origin of the fish meal, usually between 10-20%. We determined the size and shape of the lacunae in bone samples that we grinded in a pestle up to a particle size of <0.5 mm, because the particles obtained after the production of meat and bone meal are approximately of that size (11). We bleached the obtained fragments with sodium hypochlorite by immersing them for about 10 minutes and then we dried them. The preparations for analysis were made on a cover glass with droplets of Norland Optical Adhesive 65 (Norland Products, USA) where we placed a small amount of bone fragments and covered them with a cover glass. The preparations were dried by UV rays with a wavelength of 320-400 nm for 10 minutes wherewith we obtained permanent preparations. We examined the preparations with 200 times magnification.
The lacunae are divided according to their shape, into ellipsoids, stars and elongated ellipsoids. The results of the analyses were recorded as follows:
- In regard to terrestrial animals, samples containing constituents were registered as positive samples, whereas samples without constituents originating from terrestrial animals were registered as negative samples.
- In regard to the presence of fish meal, constituents obtained from fish (positive) were found in the analyzed sample, and constituents obtained from fish were not found in the analyzed sample (negative).
RESULTS
By applying the microscopic method to the animal feed samples, we performed detection in order to determine the presence of animal proteins that originate from mammals and fish. The microscopic analysis of all 421 samples, of which 115 were raw materials for the production of animal feed, 230 were concentrates for ruminant nutrition and 76 were concentrates for non-ruminant nutrition (32 concentrates for laying hens and 44 concentrates for pigs), did not provide positive results, that is, no remains of animal tissues of mammalian origin were found in any specimen (
Table 1). Whereas in 10 out of 32 (31.25%) concentrates intended for non-ruminant nutrition (laying hens), pieces of fish tissue were found. In these samples, we usually detected the presence of fish bones, gills and scales (
Fig. 1 and
2;
Fig. 6;
Fig. 7 and
Fig. 8).
The detection of ingredients of animal origin were found, such as muscle fibers (
Fig. 3) and other meat parts, cartilage (
Fig. 4), bones, fibers (
Fig. 5 and
Fig. 6), eggshells and scales (
Fig. 8). Microscopic images of animal body parts are shown below.
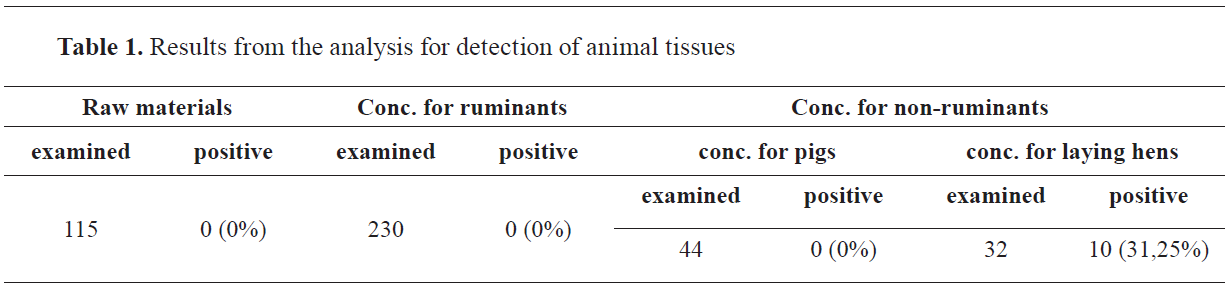
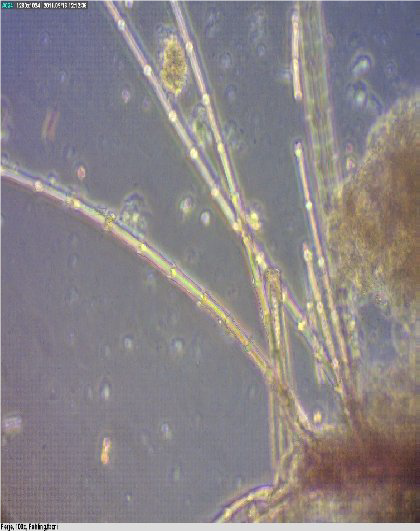 Figure 1.
Figure 1. Feathers
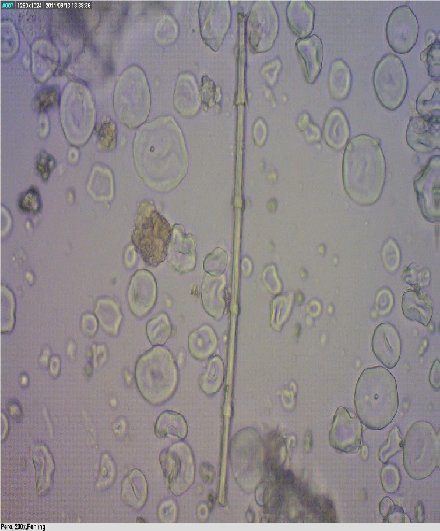 Figure 2.
Figure 2. Feather
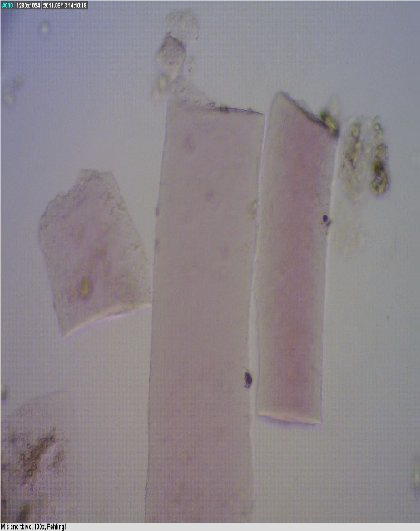 Figure 3.
Figure 3. Muscle tissue
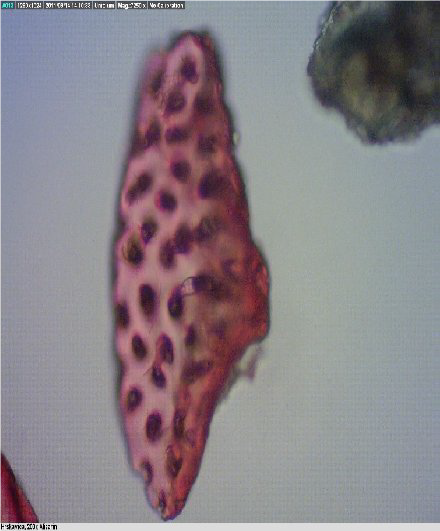 Figure 4.
Figure 4. Cartilage
Fragments of mammalian and fish bones are shown in
Fig. 5 and
Fig. 6. The shape of the cavities in mammalian bones are round, whereas in fish bones are elliptical.
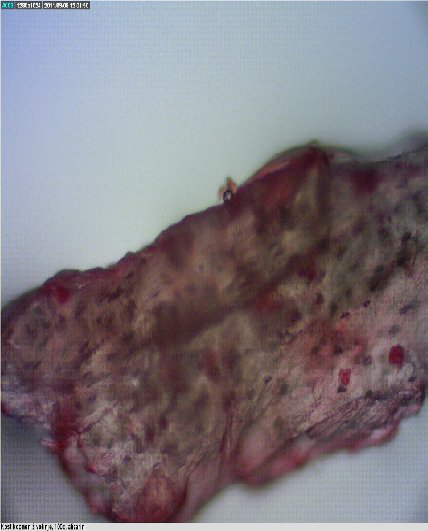 Figure 5.
Figure 5. Bone of a terrestrial mammal
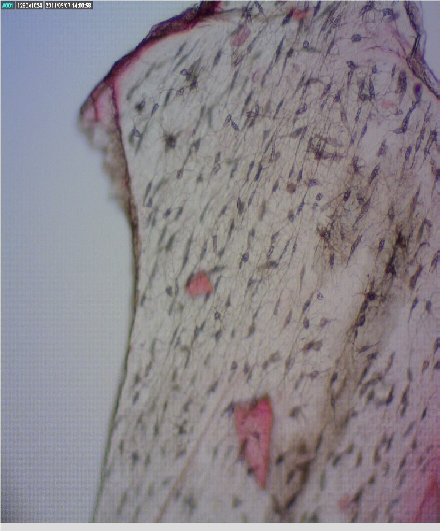 Figure 6.
Figure 6. Fish bone - lacunae
Presence of gills and fragments from fish bones were found in chicken concentrates (
Fig. 7 and
Fig. 8).
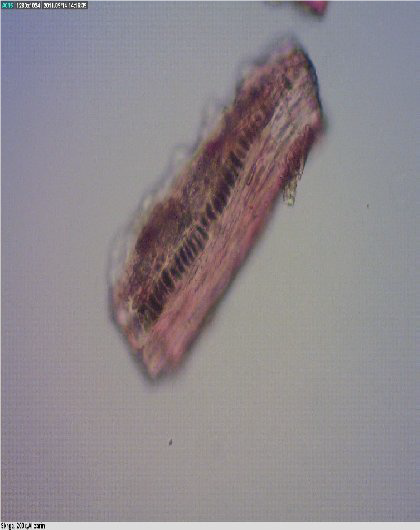 Figure 7.
Figure 7. Fish bone
 Figure 8.
Figure 8. Scale
Dense arrangement of the lacunae in the fragments of bird and pig bones is evidenced in
Fig. 9;
Fig. 10;
Fig. 11 and
Fig. 12.
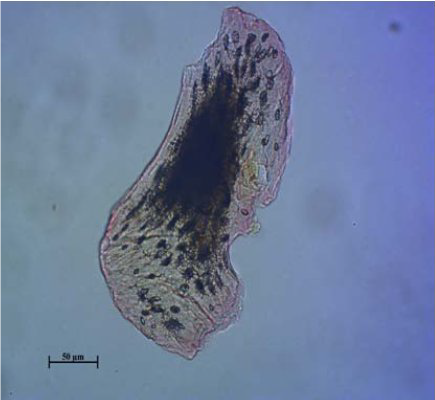 Figure 9.
Figure 9. Bird bone
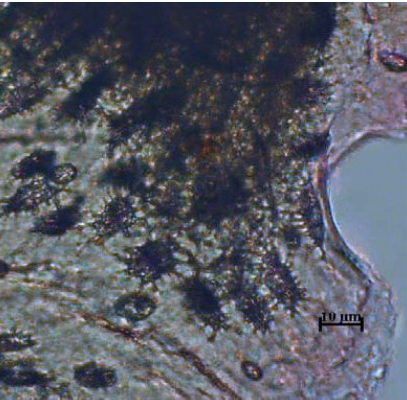 Figure 10.
Figure 10. Lacunae - bird
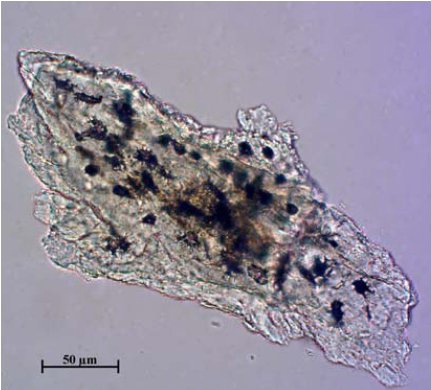 Figure 11.
Figure 11. Pig bone
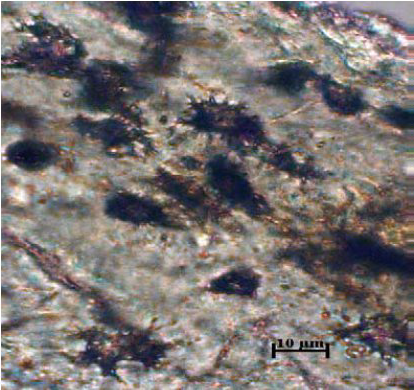 Figure 12.
Figure 12. Lacunae - pig
Longer and rarer, properly arranged lacunae with an elliptical shape are shown in cattle and sheep (
Fig. 13;
Fig. 14;
Fig. 15 and
Fig. 16).
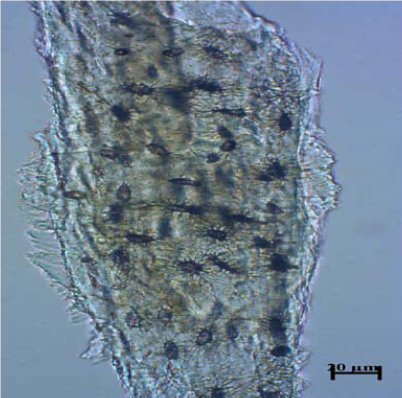 Figure 13.
Figure 13. Bovine bone
 Figure 14.
Figure 14. Bovine lacunae
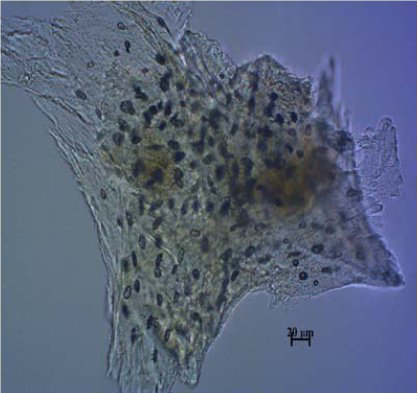 Figure 15.
Figure 15. Sheep bone
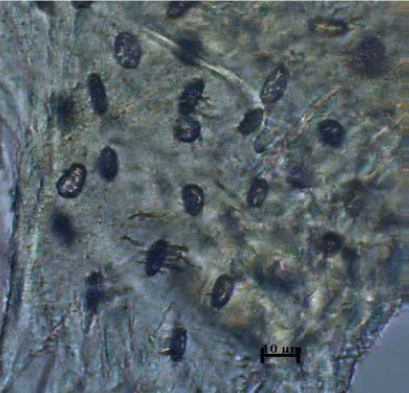 Figure 16.
Figure 16. Lacunae - sheep
Morphometric analysis of bone’s lacunae in fish, birds and mammals is presented in
Table 2. while different shapes of bone’s lacunae are shown in
Fig. 17.
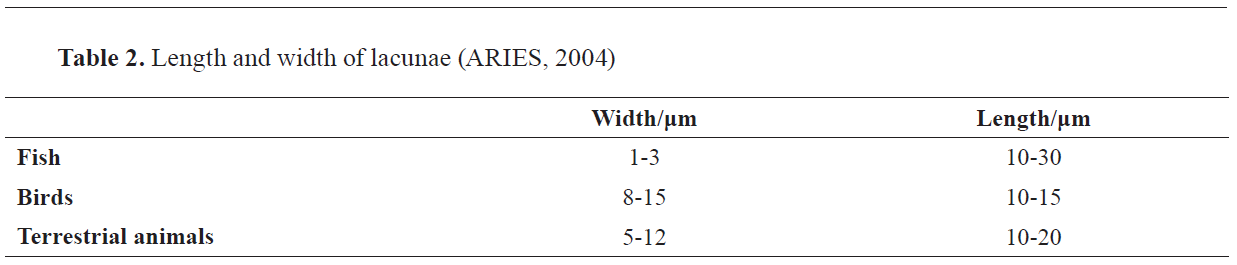
 Figure 17.
Figure 17. Lacunae shapes. Elliptical shape (a); star shape (b); elongated elliptical shape (c)
DISCUSSION
In the analyzed samples, we determined the type of animal tissue present. For the tissue detection we used the specifics on the shape and the size of lacunae and bone fragments from terrestrial animals, birds or fish. This possibility to differentiate bones from terrestrial mammals and birds has proved very useful in the control of spongiform encephalopathies in farm animals (
3).
Meat and bone meal is produced by heat treatment of animal carcasses or parts thereof, after which the carcasses or their parts are ground into a powder of 0.5 mm particle size. The meat and bone meal is usually obtained from slaughter remains or farm corpses, which means that it contains portions of all kinds of animals, such as pigs, chickens, cattle (
11). The concentrates in animal feed for farm animals are controlled for the presence of meat and bone meal by microscopically checking their composition (
17) and in an event when animal tissue is found, such food is removed. However, some animal species can be fed with fish meal. It is therefore important to identify fragments of fish bones, terrestrial mammals and birds. Fish bones have distinctly elongated, spindle-like lacunae with long branched canaliculi (
Fig. 18) (
9).
We compared the data we obtained with the data from the study by Kwiatek, and Weiner (
12), which confirmed the presence of fish meal in 156 (3.04%) non-ruminant concentrates from 5138 tested concentrates, whereby we can confirm that the presence of fish meal in our study is not low, which suggests a significant omission and entails a reduction in the quality of produced meat and eggs.
The percentage of positive samples obtained in this study is generally much lower than those presented in other papers where the number of positive results ranged between 2.9 - 93.75%. In the study (
13) conducted in North Germany, the author showed that 30 (93.75%) of the 32 examined specimens of animal feed for ruminants contained animal protein residues. The author considers that the reason for this is the cross-linking during the production of concentrates for ruminants and nonruminants or the containers used for food transport.
In Switzerland, following the introduction of the general ban on the use of animal protein in animal nutrition, the number of cases of contaminated food has decreased significantly. In 2001, only 2.9% of the samples analyzed for the presence of animal protein were positive compared to 14% in 2000 (
12).
The efficacy of microscopic detection of animal tissues has been confirmed in the study (
14) providing a result of ring testing performed in 19 laboratories, on animal feed samples contaminated with meat and bone meal at the level of 3%, 0.5%, 0.1 % and 0.02%. The obtained results show only one false negative result on a sample with a contamination level of 0.02%.
In the study (
15), the authors presented results for the presence of mammalian animal tissues in fish meal although the meat and bone meal was added at a very low concentration of 13%. According to these authors, the lowest concentration of meat and bone meal added to fish meal, which can be detected, is 0.1%.
Many of the studies published so far in the EU describe the microscopic analysis procedure and the results of the examined animal feed. The results show that this technique provides the detection of ingredients of animal origin such as muscle fibers (
Fig. 3) and other meat parts, cartilage (
Figure 4), bones, fibers (
Fig. 5 and
6), eggshells. and scales (
Fig. 8). By using binding and coloring reagents, it is possible to see constituents of animal origin in animal feed. The staining with cystine reagent and paraffin oil as a binding reagent was particularly useful. Bones of mammals and birds can also be differentiated (
16,
17).
The arrangement of the lacunae in the fragments of bird and pig bones is very dense, giving the impression that they have black surfaces (
Fig. 9-12). In cattle (
bovinae) and sheep, the lacunae are longer and rarer, properly arranged with an elliptical shape (
Fig. 13-16).
In Table 2 the values regarding the length and width of all types of lacunae per species are displayed. The data on fish, birds and terrestrial mammals is retrieved from the ARIES software, which is being used as an auxiliary tool in identifying bone fragments in microscopic analysis. The difference in the size of lacunae among birds, terrestrial mammals and fish is evident.
The microscopic images show different parts of the animal’s body.
Fig. 5 and
Fig. 6 show fragments of mammalian and fish bones, the typical difference being in the shape and size of the cavities, which in the mammalian bones are round, whereas in fish bones they have an elliptical shape. In fish bones, more elliptical-shaped cavities can be observed, which form a network around the lacunae. The absence of these specific features suggests the absence of animal parts in the animal feed.
By microscopic analysis, in addition to the expected plant components, in the chicken concentrates we found the presence of gills and fragments of fish bones (
Fig. 7 and
Fig. 8). Based on the experimental data, this study confirms the authors’ statement (
9) that there is no conditional approach that will meet all requirements and that the methods complement each other. Although microscopy is a powerful method for detecting even the smallest trace of meat and bone meal in animal feed, other suitable methods are combined for special purposes in order to obtain the greatest volume of information possible.
CONCLUSION
With the microscopic method for detection of prohibited animal tissues in animal feed, we found that in the animal feed concentrates for ruminants, a total of 230 samples, residues of animal tissues of mammalian origin were not found in any sample. During the control of animal feed (concentrates for non-ruminant animals), by applying the microscopic method, we examined 44 concentrates for pigs and 32 concentrates for laying hens. In addition to the expected plant components, we found the presence of fish bones in the concentrates for laying hens. Of the examined 32 concentrates, we found fish residues in 10 concentrates for laying hens, which is 28.57%.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
This research was supported by the Faculty of Veterinary Medicine – Skopje.
AUTHORS’ CONTRIBUTION
RCN conceived and designed the study and wrote the manuscript. RCN, AA, KB and RU performed the experiments. VPT contributed to the final version. SG gave critical revision.

 10.2478/macvetrev-2021-0021
10.2478/macvetrev-2021-0021


















