The study was planned to evaluate the use of laparoscopy for the diagnosis and retrieval of abdominal cysts in sheep and goats. The abdominal cysts were located in 10 of 135 adult, healthy small ruminants by using ultrasonography (USG). Percutaneous Puncture-Aspiration-Injection-Reaspiration (PAIR) technique was used for six animals. Thirty animals, including four diagnosed with abdominal cysts by USG, were subsequently subjected to laparoscopy under diazepam sedation (0.1 mg/kg, IV) and lumbosacral epidural regional anesthesia using 2% lignocaine hydrochloride (1.0 ml/5 kg). The animals were restrained in dorsal recumbency and two-port paramedian laparoscopy was performed. Abdominal cysts were located in seven animals (one cyst/animal). The cysts were grasped carefully, lifted close to the abdominal wall, and evacuated percutaneously under laparoscopic vision. The collapsed cysts were then retrieved. In two animals enlarged ports were sutured, treated with an antibiotic and an analgesic, resulting in uneventful healing. Five cysts were nonparasitic, two were parasitic, one extracted from a sheep, and one from a goat. In conclusion, laparoscopy has higher diagnostic accuracy in detecting unattached abdominal cysts in sheep and goats compared to USG. It is also a reliable, minimally invasive, and safe procedure for cyst retrieval. However, a larger-scale study is necessary for ascertaining long-term complications and the recurrence rate.
Infectious and non-infectious diseases of the abdominal organs are common in small ruminants. In these animals, the physical examination can be augmented using ancillary tests, assessment of clinicopathological parameters, and the use of imaging modalities. The use of various imaging techniques has improved the diagnosis and treatment of focal as well as diffuse internal diseases (
1). Ultrasonography (USG) is well suited for characterization of the forestomach and intestines, presence of masses, intraluminal and free abdominal fluid, and lesions within the parenchyma of abdominal viscera (
2). Cystic focal lesions that predominantly remain asymptomatic are coincidently noticed on abdominal imaging (
3). USG is useful for monitoring zoonotic cystic Echinococcosis in small ruminants, but it is not optimally used (
4).
In human medicine, surgery has remained the mainstay for managing abdominal (hydatid) cysts, although chemotherapy and the PAIR (Puncture, Aspiration, Injection of scolicide, and Reaspira tion) are popular modalities (
5). The laparoscopic technique which is a minimally invasive, safe, and effective approach is currently preferred over the traditional laparotomy (
6). In developing countries, the university multispecialty veterinary hospitals have started procuring and using modern diagnostic imaging and treatment facilities for farm animals. The livestock owners are now even more concerned about animal welfare. In small ruminants, the use of laparoscopy was earlier restricted to various reproductive technologies (
7), but its application is now extended for treatment (
8, 9). As per the available literature, the laparoscopic diagnosis and retrieval of the abdominal cysts in small ruminants have not been reported yet. A prospective preliminary trial was therefore planned to assess the feasibility of this modality in managing loose abdominal cysts in sheep and goats.
MATERIAL AND METHODS
The study was recommended by the Institutional Animal Ethics Committee (IAEC) registered under no. 1809/CPCSEA and subsequently approved by the Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), Government of India, New Delhi.
A total of 135 sheep and goats (aged 3-4 years) were subjected to USG examination to identify focal abdominal lesions. Thirty animals (20 ewes and 10 does) undergoing various laparoscopic reproductive interventions were additionally checked on USG for determining its non-pregnant status and for detecting focal or diffuse lesions. Standard procedure using a real-time B-mode USG machine (Esaote My lab 40 vet) with linear and sector transducers (5-10 MHz) was used for all the animals.
In 10 animals (eight sheep and two goats) with abdominal cysts detected with USG, two were located on the liver and four percutaneously accessible cysts which were managed by PAIR technique (
10). The remaining animals (three sheep, and one goat) were subjected to laparoscopic cyst retrieval.
All the animals (n=30) were subjected to laparoscopy one week following USG. Preoperatively, they were fasted for 30 to 32 hours. The wide abdominal area caudal to the xiphoid was clipped and shaved. Administration of diazepam (0.1 mg/kg, IV) was followed by lumbosacral epidural regional anesthesia using 2% lignocaine hydrochloride (1.0 ml/5 kg).
The animals were restrained in dorsal recumbency in Trendelenburg position (30° to 35°) in a cradle. The shaved area was scrubbed and isolated using sterile drapes. Laparoscopic instruments of Karl-Storz, DmbH, Germany, including Hopkin II straight forward (0° viewing angle of vision) telescope having 5.0 mm diameter and 29.0 cm length were used. The assembly was provided with cold light fountain xenon, with 175-Watt lamp and 4.5 mm fiber optic light cable. The Veress needle was inserted in the paramedian area, 2.0 cm cranial to the mammary glands and 3.0 cm to 4.0 cm lateral to the
linea alba. Using filtered air, pneumoperitoneum (12 mmHg) was established. After giving a nick (1.0 cm) in the skin, in the contralateral paramedian area (in level with the Veress needle insertion site), a trocar-cannula (6.0 cm diameter) was inserted to create the first port. The abdomen and pelvis were explored meticulously by inserting the laparoscope (5.0 mm) through the port. The cradle was also tilted reverse Trendelenburg for maximum possible exploration.
After a cyst in an animal was located, the second port was established by replacing the Veress needle with a threaded trocar-cannula (6.0 cm diameter) (
Fig. 1). All animals with abdominal cysts were injected dexamethasone (8.0 mg) intravenously. The cyst was grasped carefully with laparoscopic forceps, passed through the second port, and lifted close to the abdominal wall (
Fig. 2).
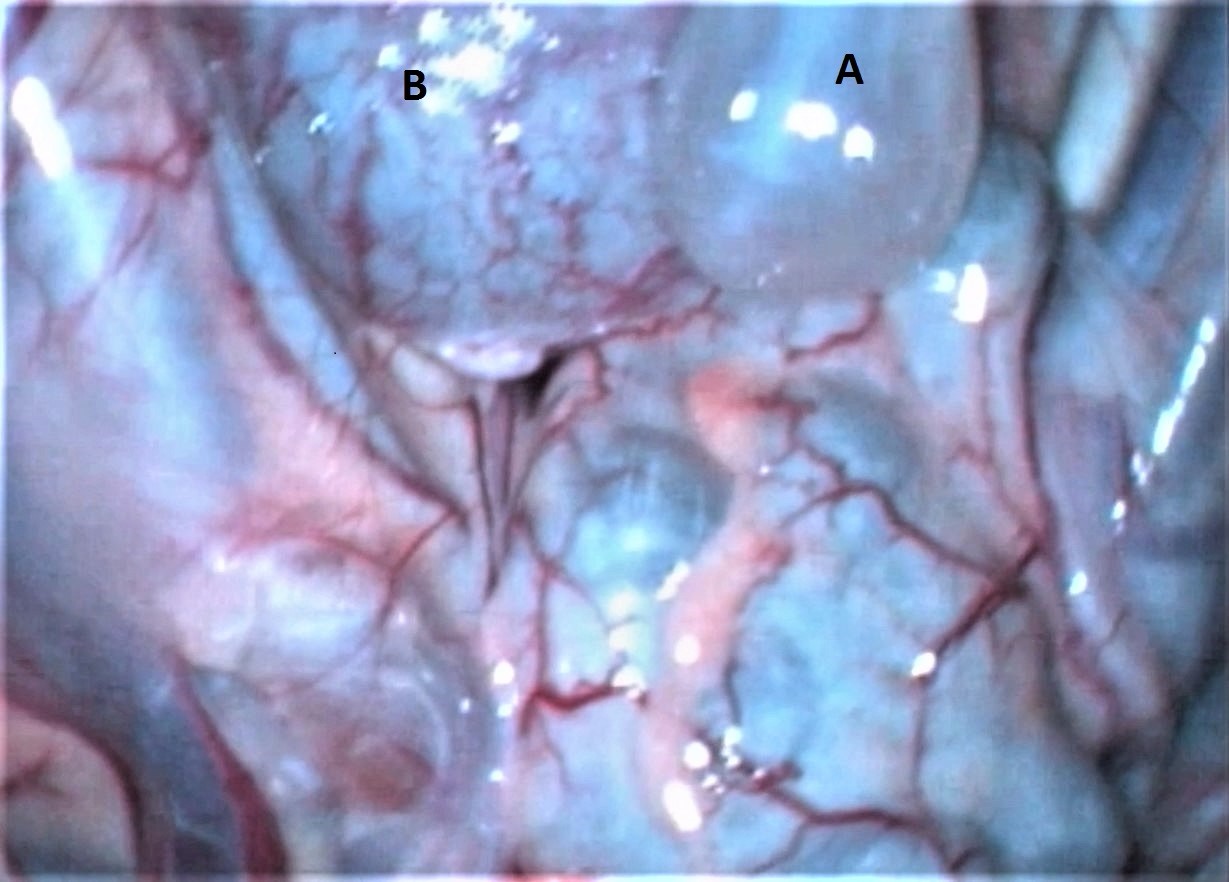 Figure 1.
Figure 1. Laparoscopic view of an abdominal cyst: A) cyst, B) cecum
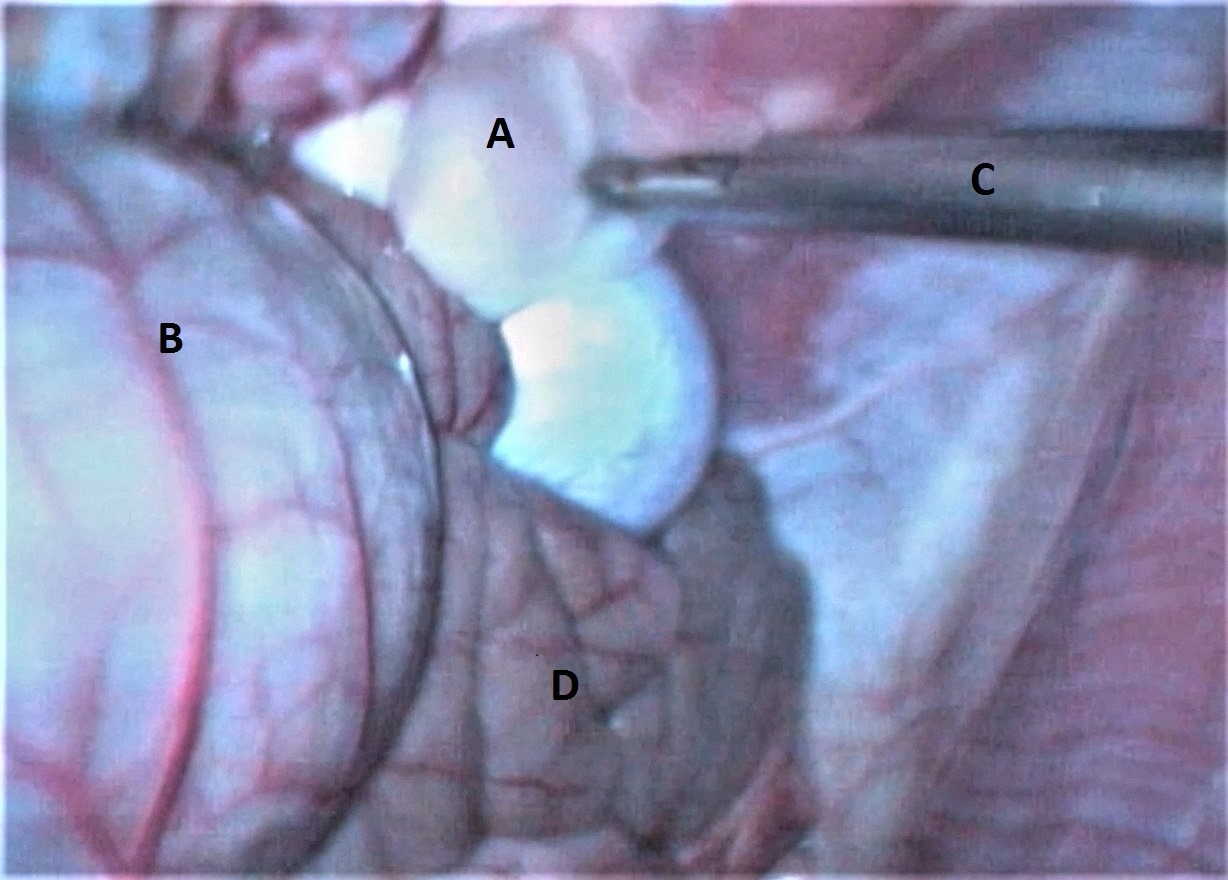 Figure 2.
Figure 2. Laparoscopic view of an abdominal cyst being held by a grasping forceps: A) cyst, B) cecum, C) laparoscopic cannula and grasping forceps, D) small intestines
An 18-G hypodermic needle attached to a dispovan (10.0 ml) was passed percutaneously into the cyst and the contents were evacuated carefully under continuous laparoscopic vision.
The collapsed cyst wall was subsequently pulled out of the abdomen by the forceps (
Fig. 3).
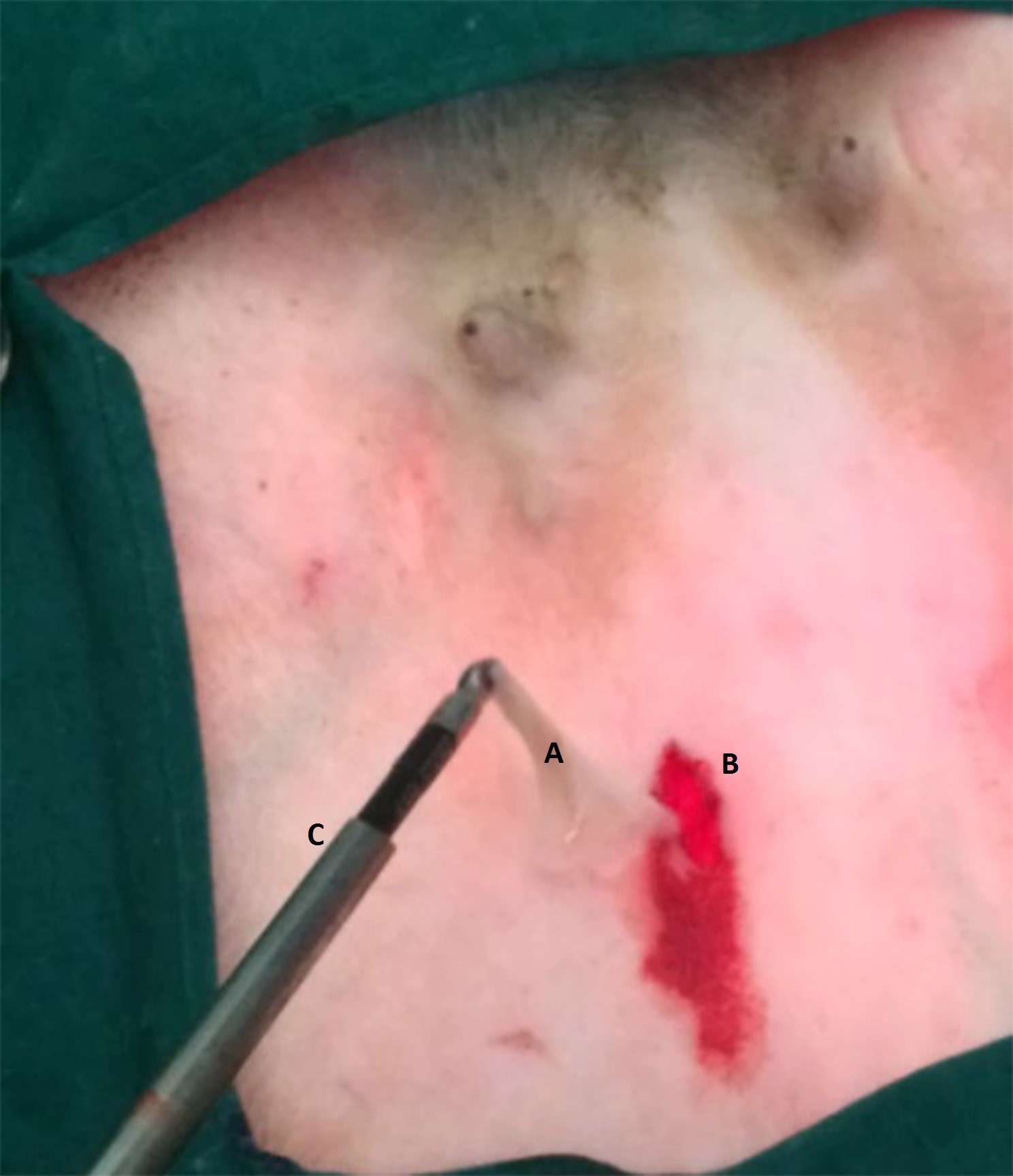 Figure 3.
Figure 3. Retrieved evacuated cyst wall: A) empty cyst after retrieval from abdomen, B) port site, C) laparoscopic grasping forceps and cannula
In two animals, the port had to be enlarged by 1.0 cm additional incision in the craniocaudal direction, the cannula pulled out along with the grasping forceps, and the cyst wall retrieved without spillage of its contents. The enlarged ports were closed using chromic catgut (No 1) in a simple continuous pattern followed by cutaneous interrupted horizontal mattress sutures using nylon (No 1).
All the port sites were treated with a fly repellentantiseptic cream. Antibiotics (amoxycillin-dicloxacillin, 0.5 g, IM) and analgesic (meloxicam, 0.5 mg/kg, SC) were given in a single dose. Routine postoperative care of the port sites was continued for two weeks.
The recovered cyst contents and the cyst walls were sent for parasitological and histological examinations. After evaluating the gross morphology, the scolexes present in the cyst fluid were crushed between two slides and examined under the microscope. The cyst material found negative on parasitological evaluation were preserved in 10% formalin solution. They were subsequently subjected to paraffin embedding. Sections of 5-6 μ thickness were stained using H&E and Mason`s Trichrome according to the standard procedure (
11).
RESULTS
Cysts were identified in 10 animals (eight sheep and two goats) on USG examination. They appeared as globular or oval anechoic structures with smooth margins in the mid-abdomen area adjacent to the viscera. Compartmentalization was not detected in any cyst (
Fig. 4). Four of these animals not suitable to PAIR procedure were included in this study.
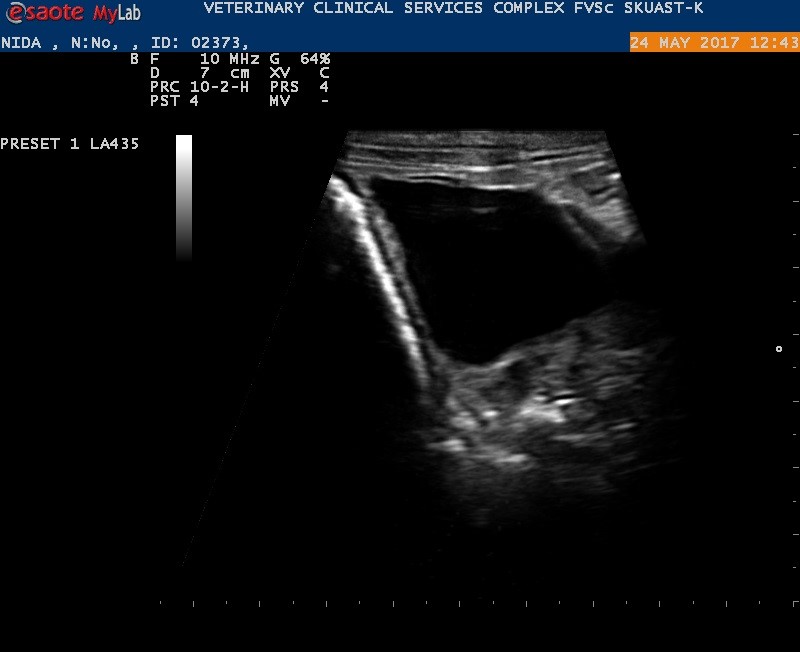 Figure 4.
Figure 4. Ultrasonographic view of the abdominal cyst
The sedation in combination with the lumbosacral and epidural local anesthesia produced satisfactory regional anesthesia for a sufficient duration of time in all the animals included in this study.
The produced pneumoperitoneum afforded sufficient space for satisfactory visualization and manipulation during the laparoscopy procedure. The abdominal and pelvic contents were easily visualized in all the animals by orienting the laparoscope in different directions. Trendelenburg and reverse Trendelenburg postures were aiding the examination.
The cysts identified in four ewes on preoperative USG were successfully located during the laparoscopy (
Fig. 1). Three more animals (two sheep and one goat) that were negative on USG were also found to have abdominal cysts.
The cysts were located in the abdominal cavity of the animals as round/oval structures with transparent walls and colorless fluid contents. They were not attached to any of the viscera within the abdominal cavity and would periodically go partially or completely out of vision whenever displaced or covered by the intestines showing normal motility.
The laparoscopy was helpful in visualization (with magnification) of the abdominal and pelvic organs and also the normal gastrointestinal motility. The cysts were the only focal lesions located on USG and laparoscopy. They were lifted, aspirated under laparoscopic vision within the abdomen, and the cyst walls were retrieved without rupturing or spilling of its contents.
All of the animals survived without complications. The cutaneous sutures were removed 10 days following the laparoscopy. All the port sites showed uneventful healing. Subcutaneous emphysema was noticed in the immediate post laparoscopy period in one sheep which was resolved within three days.
Two of the seven recovered cysts (one each from sheep and goat) were parasitic. On gross examination, a single scolex was found attached to the thin cyst wall. The rostellum of the scolex possessed two rows of alternating large and small hooks (
Fig. 5).
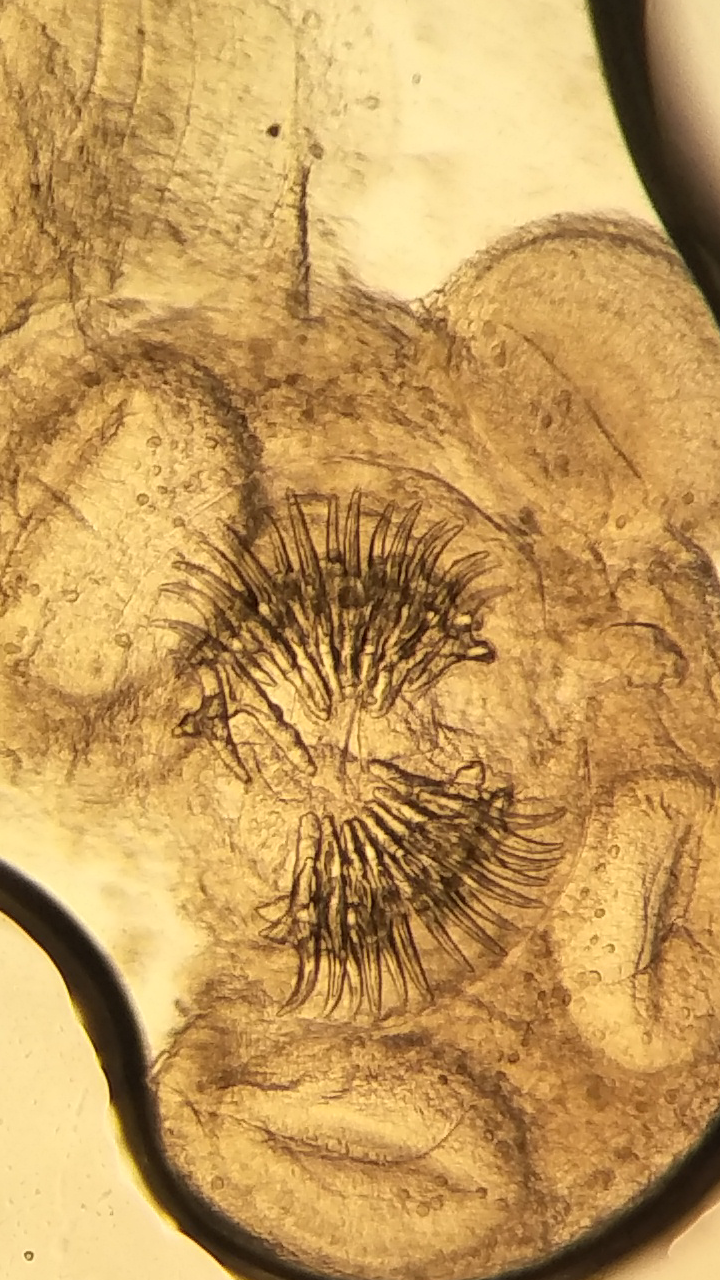 Figure 5.
Figure 5. The parasitic cyst with rostellum of the scolex showing two rows of alternating large and small hooks (40x)
The histological examination of the five remaining nonparasitic cysts revealed epithelial cell columns, thick sub-epithelial tissue, and dentate appearance (
Fig. 6).
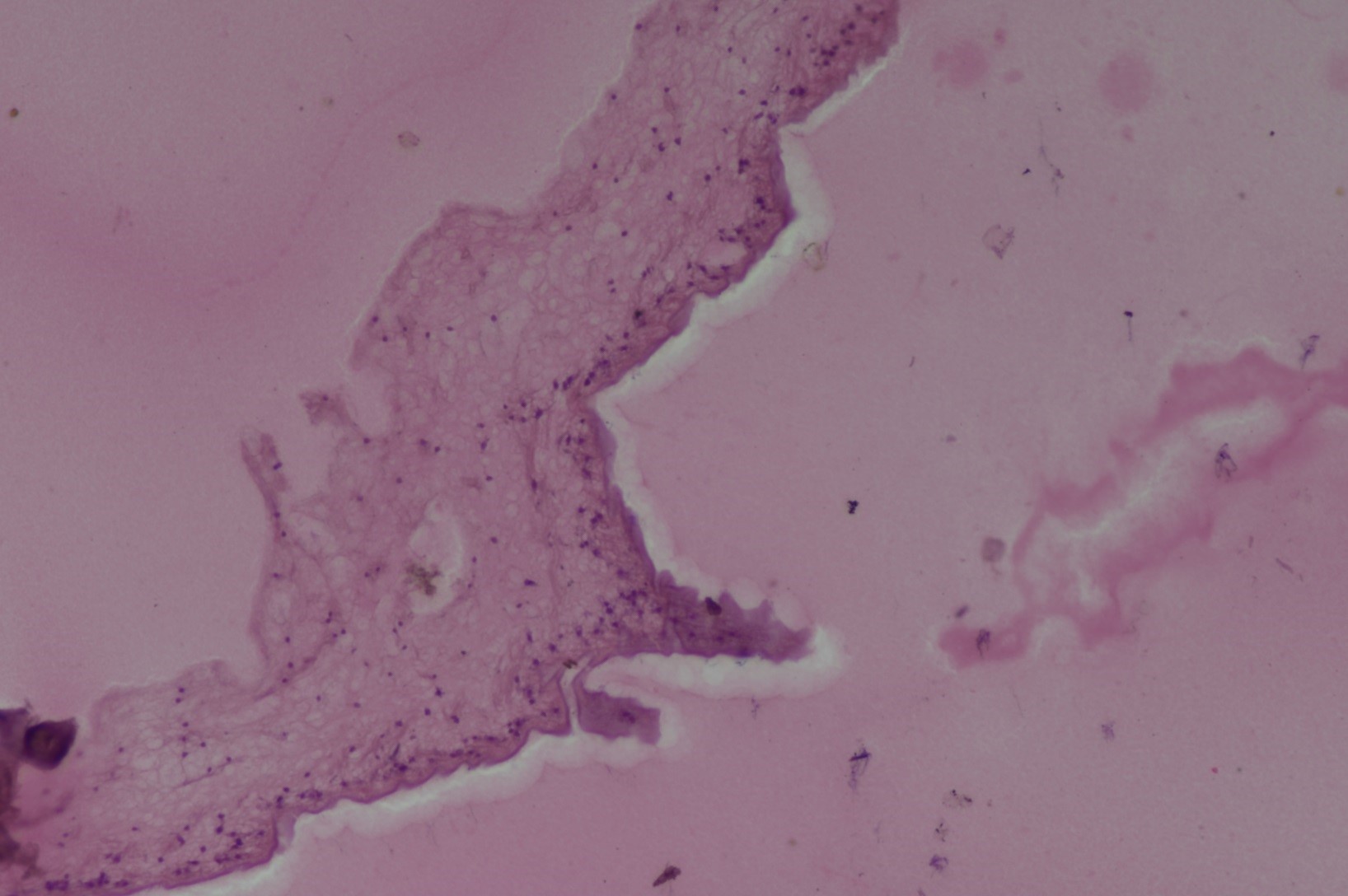 Figure 6.
Figure 6. Histological view of a nonparasitic cyst lining/wall (H&E; 20x)
DISCUSSION
USG and laparoscopy have revolutionized medicine due to their use in disease diagnosis and treatment (
12, 13, 14). USG is a quick, non-invasive, and well-tolerated technique for the diagnosis of cystic disease (
4, 13). The patients with abdominal cysts are usually asymptomatic and are incidentally diagnosed on USG (
15). In small ruminants, the use of laparoscopy was restricted to various reproductive technologies, but this modality has started receiving attention in disease diagnosis as an alternative to exploratory laparotomy and treatment (
12).
The animals in this study were fasted for at least 30 hours before laparoscopy. Preoperative fasting is mandatory before any laparoscopic procedure. In sheep and goats, 36 to 48 hours of fasting decreases the gastrointestinal contents and reduces intestinal peristalsis (
12). Consequently, the intraoperative accidental organ punctures and excessive pressure on the diaphragm are avoided. It also makes exploration of various abdominal/pelvic structures easy.
Standardized sedation and lumbosacral regional anesthesia protocol were used for this study (
16, 17, 18). Xylazine sedation with local infiltration anesthesia was reported earlier with satisfactory anesthetic effect (
19). Laparoscopy was safely performed under general anesthesia using pentobarbital or combinations of promazinepentobarbital, atropine-xylazine-thiopental, or ketamine-xylazine (
9, 20).
For the experimental animals in the current study, the primary port was created in the paramedian area. Trans-umbilical insertion of the laparoscope for visualization of the abdominal cavity may also be adopted satisfactorily in goats (
21).
By utilizing the laparoscopy, three cases were diagnosed in addition to the four animals detected by USG. In a study involving 284 goats and 16 sheep, 10.3% showed cysts on USG and 15.3% on post-mortem examination. In the same trial, 21 animals (8.26%) were found to have cysts on post-mortem examination which were falsely identified as negative, and six (2.4%) others as positive on USG (
13).
Solitary cysts were present in all seven animals. In one study involving humans, the hepatic hydatid cysts were solitary in 53%, two in 27%, and more than two in the remaining 20% of patients (
22).
The animals with abdominal cysts were injected corticosteroid to prevent anaphylaxis. A human patient treated laparoscopically for two cysts developed anaphylactic reaction intraoperatively but was managed successfully with fluids, ephedrine, and dopamine (
23).
The animals had minimal exposure of the abdominal/pelvic cavities to the outside atmosphere and were subjected to minor visceral handling during laparoscopy. The antibiotics and the analgesic were therefore injected only once. Following laparoscopy, such drugs are routinely required for the first 24 hours (
18, 22).
Two of the seven recovered cysts were parasitic. The rostellum in
Taeniidae was armed with a double row of large and small hooks with a characteristic shape (
24). In small ruminants, the
Cysticercus tenuicollis is an important disease that causes great economic loss due to organ condemnation. Omentum and mesentery are the predominant predilection sites (
25, 26).
The histological features of the non-parasitic cysts were similar to the previously reported descriptions (
27).
CONCLUSION
The laparoscopy can diagnose freely roaming abdominal cysts in a higher number of sheep and goats than USG. It is also a reliable, minimally invasive, and safe procedure for cyst retrieval. The long-term complications and the recurrence rate should be assessed by a larger-scale study.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The laparoscopic instruments were procured by Division of Animal Biotechnology under Indian Council of Agriculture Research (ICAR), NAIP project no. 20029101. Thanks are due to the Vice-Chancellor for providing internal university facilities and resources.
AUTHORS’ CONTRIBUTIONS
NH and MF made the concept, design and draft version of the work. NH, MF, MAG, and RAS performed the anaesthesia and laparoscopy in animals. HA and NH made USG of animals. IA made parasitological evaluation of cysts. BK, MM and NH were responsible for the histopathological evaluation of cysts. MF supervised the whole work and made the final approval of the version to be published.

 10.2478/macvetrev-2021-0022
10.2478/macvetrev-2021-0022





