Stress can be a reason for some physiological and biological disorders in the body. The antioxidative defense system is necessary for the maintenance of redox homeostasis in the organisms. Alkaline water (AW) is in the focus of the scientific interest due to its antioxidative effect. The treatment with AW and sodium ascorbate (SA) is expected to have potential preventive effect on the organism to hyperthermic stress. The aim of this study was to investigate the effect of AW and SA on glucose and cortisol levels during acute hyperthermic stress, in white female Wistar laboratory rats. The rats were divided into three groups, each having 10 subjects. They were exposed in hyperthermic conditions (41˚C) for 80 min, in 21 consecutive days in order to induce oxidative stress. The first group received drinkable water (control group), the second AW, and the third, AW and SA. Plasma glucose levels were determined by colorimetric method. Cortisol level was measured by the enzyme-linked immunosorbent assay method (ELISA). The means were compared using the Tukey test. Differences were considered significant at a level of p<0.05. Our results showed that levels of glucose and cortisol were significantly higher in the group treated with AW on the 21st day after treatment (p<0.0001), but not on the 7th and 14th day as compared to the control group. Also, co-treatment of animals with AW and SA had significantly increased the levels of glucose and cortisol on the 21st day after treatment, indicating a synergistic effect. In conclusion, the individual action of AW or in synergism with SA caused a high protective effect on oxidative damage in white Wistar laboratory rats.
Alkaline water (AW) has the ability to alkalize the organism and to suppress oxidative stress related diseases, so it has attracted an increased attention over the last decade. Also, it has the ability to scavenge reactive oxygen species (ROS) (
1), reduce blood glucose levels, exhibit various antineurodegenerative disease effects (
2), accelerate growth, and promote metabolism (
3). AW helps in disease prevention because it neutralizes free radical levels in living organisms. (
4). It was reported that AW has protective effect on DNA from oxidative damage by scavenging ROS in vitro (
1) and stimulates glucose uptake in muscle cells and adipocytes (
5). Kim and Yokoyama have reported that the blood levels of glucose and lipid peroxide were reduced due to AW administration to Goto Kakizaki (GK) rats (
6). It was also reported that the activity of hexokinase, which is a pivotal enzyme inducing the reduction of blood glucose levels, can be substantially increased by AW (
7). AW can suppress the oxidative damage induced by alloxan (a type 1 diabetes inducer) in cells and in alloxan induced type 1 diabetes model mice (
8, 9, 10, 11). The imbalance between production and accumulation of ROS in cells and tissues is the cause for oxidative stress which is determined by the body’s detoxification ability. ROS can play several physiological roles (i.e., cell signaling), and they are by-products of aerobic metabolism. Moreover, the ROS production is greatly increased by ionizing radiations, UV, pollutants, and heavy metals which are environmental stressors and xenobiotics. This causes the imbalance that leads to cell and tissue damage (
12).
Increased environmental temperatures induce oxidative stress in animals by increasing metabolic activity and oxygen consumption (
13). The formation of free radicals increases significantly in conditions of hyperthermia (
14). Vitamin C is a powerful non-enzymatic antioxidant capable of reacting with a wide range of biological oxidants, and it is a cofactor for a family of biosynthetic and gene regulatory enzymes. Vitamin C can accumulate in phagocytic cells, like neutrophils. It can strengthen chemotaxis, phagocytosis, generation of ROS, and microbial killing (
15). Sodium ascorbate is a form of Vitamin C which has sodium components, lower acidity level, higher intestinal absorption, and higher body retention time. A higher oral intake of vitamin C can be potentially beneficial, by inducing higher reduction of ROS and lower oxidative damage in tissues (
16). The chemical structure of vitamin C is similar to the glucose so it can replace it in many chemical reactions. Thus, it is involved in preventing the nonenzymatic glycosylation of proteins (
17). Conflicting results can be found in available literature, regarding supplementation of vitamin C and improvement in blood glucose level and glycosylated hemoglobin (HbA1c) (
18). The level of cortisol during stress was reported to be increased in wild, farm, domestic, and laboratory animals (
19, 20). Cortisol is synthesized from cholesterol and has a variety of effects on different functions throughout the body. As an end product in the hypothalamic-pituitary-adrenal (HPA) axis, cortisol is mainly secreted in reaction to stress. It also has a key role in normal physiology (
21, 22). The concentration of vitamin C in the blood increases when the HPA-axis is stimulated. This observation was first noticed in 1962 by Lahiri et al. (
23, 24). There is an inverse correlation between the ability of an animal to endogenously produce vitamin C and the cortisol response under stress (
15, 25).
MATERIAL AND METHODS
Animals and experimental designAll experimental procedures were conducted in accordance with the Guiding Principles for Care and Use of Laboratory Animals accepted by the Macedonian Center for Bioethics. All protocols were approved by the Animal Ethics Committee of the University “Ss. Cyril and Methodius”, Skopje, R. N. Macedonia (UP-1. NO. 2401-592) in accordance with the International Guiding Principles for Biomedical Research Involving Animals, as issued by the Council for the International Organizations of Medical Sciences. Anesthetics were applied in accordance with the EC Directive 86/609/EEC. An intraperitoneal injection of thiopental sodium (Rhone-Poulenc Rorer Limited, Nenagh, Co Tipperary, Ireland) was used to anesthetize the animals, 50 mg/kg b.wt. Wistar female rats (n=30), 6 months of age, weighing 210-300 g, were used for all experimental protocols. They were maintained on a 12:12 light-dark cycle and fed with standard rat chow and water ad libitum, at a thermoneutral temperature of 22 ºC. The experiment was conducted in 21 consecutive days.
Experimental protocolThe first group of animals (control group, n=10) was kept under conventional laboratory conditions and it received drinkable water during the entire experimental period.
The second group of animals (n=10) received AW by intragastric infusion.
The third group of animals (n=10) received AW and SA by intragastric infusion.
Intragastric portions of 2 ml were applied to each animal every day in the morning during 21 consecutive days. Blood samples (1.5 ml) were taken by tail incision, on the 7th, 14th, and 21st day of the experiment, for glucose and cortisol levels assessment. Blood serum was obtained by centrifugation at 1500 rpm and was frozen at -80 ºC, 5 minutes following sampling. On the 21st day, five hours after the last intragastric portion, animals were exposed to a hyperthermic conditions until they reached a stage of secondary hyperthermia (body temperature of 43 ºC). The exposure was performed individually in air chambers at 40±1 ºC, for 80 minutes. Then, the rats were sacrificed by subcutaneous administration of sodium thiopental. The tissues were perfused in 0.9% NaCl and instantly frozen in liquid nitrogen. All isolated materials were stored at -80 ºC until further analysis.
Determination of serum glucoseColorimetric method was used to determine plasma glucose. The aldehyde group of glucose was condensed with ortho-toluidine in glacial acetic acid. When heated, the emitting green color was measured at 630 nm which was then used to determine the glucose concentration expressed in milligrams per deciliter in 100 ml of blood.
Determination of serum cortisolThe Cortisol ELISA KIT protocol (ABNOVA, KA1885) was used to estimate cortisol. This is a solid phase ELISA based on the principle of competitive binding. It used a polyclonal rabbit antibodies directed against the cortisol molecules. The endogenous cortisol competes with a cortisolhorseradish peroxidase conjugate. Serum cortisol was analyzed in accordance with the manufacturer’s instructions. On the 96-well microtitre plate 50 μl of sampled serums from rats or cortisol standards (0.1–30.0 ng/ml) were transferred in the appropriate wells. The enzyme-conjugate (50 μl) was added in each of the wells, ensuring thorough mixing. We incubated the plate at room temperature with gentle mixing for 60 min. The contents of each well were aspirated. Then, four rinses with 300 μl wash solution were applied. In each well 200 μl of substrate was added. It was incubated at room temperature for 30 min. With the addition of 50 μl stop solution, the reaction was stopped. The absorbance was immediately read at 450 nm. All standards, controls, and samples were analyzed in duplicates. The results were calculated using a 4-parameter logistics curve fit.
Statistical analysisVariables were expressed as mean values ± standard deviation (SD). One way ANOVA was used to compare group means. Also, two-sample t-test and Principal Component Analysis (PSA) were used. The means were compared using the Tukey test. The level of significance was considered at p<0.05. The statistical software package Addinsoft (2021) XLSTAT statistical and data analysis solution, New York, USA, (
https://www.xlstat.com) was used for the analyses.
RESULTS
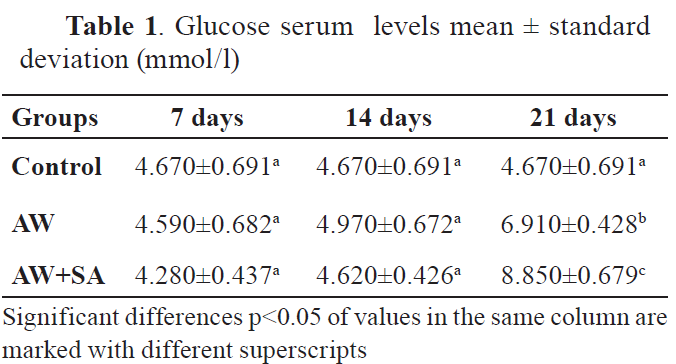
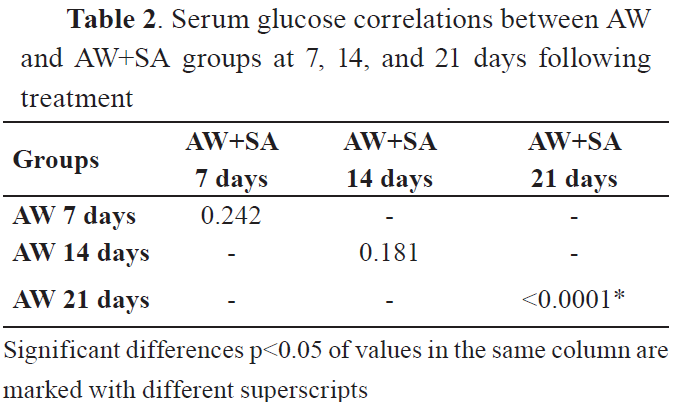
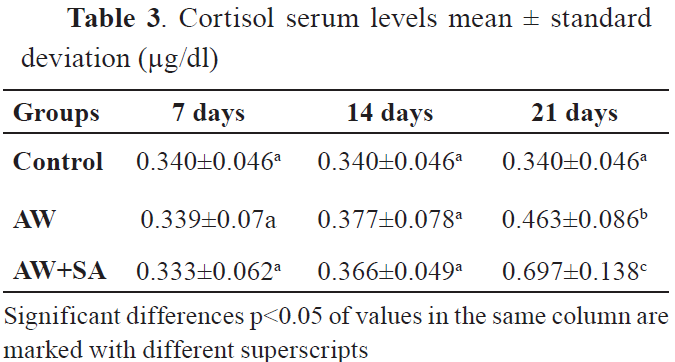
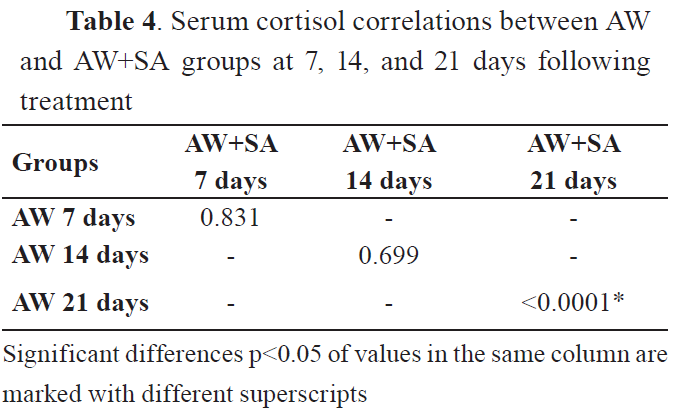
All comparisons showed significant differences after 21st day of treatment. However, the correlation matrices showed none or small positive correlations. Significant correlations were observed between values in the control group after the 21st day. The serum glucose and cortisol levels are shown in
Table 1 and
Table 3. The comparison of glucose and cortisol serum levels among groups is shown in
Tables 2 and
4.
GlucoseThe results showed that the glucose levels were higher in the group of animals treated with AW at day 21st after treatment (p<0.0001), but not at day 7th and 14th, compared to the control group. The group treated with AW and SA had significantly higher glucose levels at 21st day after the treatment.
CortisolThe results showed that levels of cortisol have increased in the group of animals treated with AW at day 21st after treatment (p<0.001), but not at the 7th and 14th day as compared to the control group. Also, co-treatment of animals with AW and SA significantly increased the cortisol levels on the 21st day (p<0.0001).
DISCUSSION
It was demonstrated that oxidative stress is associated with a variety of disorders including diabetes, hypertension, neurodegenerative diseases, and atherosclerosis (
26). Diabetes is one of the major metabolic disorders, characterized by hyperglycemia, inflammation, and insulin resistance (
27). These complications are believed to be related to ROS and oxidative stress. The use of AW was suggested to alleviate these effects. It was shown that by activating hexokinase, the administration of AW to rats decreased the concentration of blood glucose and lipid peroxide levels (
7). The effect on glycemia and lipidemia was demonstrated in OLETF rats (a model of spontaneous type 2 diabetes) (
28). During 32 weeks of supplementation, AW stimulated growth, decreased blood glucose, cholesterol, and triglyceride concentrations by 10%-20%, compared to the control rats supplemented with tap water. Moreover, Li et al. (
8) have concluded that AW can prevent alloxan induced apoptosis in vitro. The effect can be applied in order to suppress diabetes in experimental animals by feeding for prolonged periods of time (
11). Furthermore, the results of another study from testing the effectiveness of AW in the group of streptozotocin induced mice, showed that blood glucose levels can be significantly reduced with AW. Also, glucose tolerance in diabetic mice can be improved (both models mice that were genetically diabetic) (
29). These data suggest that AW can be used as an effective antidiabetic drug.
The results of our study are in accordance with the previous studies, which report that such properties of AW are shown in the second group, where acute hyperthermic exposure on the 21st day significantly (p<0.001) increased glucose levels compared to the control group. Also, co-treatment of animals with AW and SA significantly increased the levels of glucose at 21st day after the treatment.
Vitamin C can replace the glucose in many chemical reactions due to their similar chemical structure. Thus, it is effective in preventing nonenzymatic glycosylation of proteins (
17). Sreemantula et al. have demonstrated that the L-ascorbic acid has produced hypoglycemic activity in a dose-dependent manner during a diabetic condition (
30).
An imbalance in physiological responses can be induced by stress, which is associated with the activation of the hypothalamo-pituitaryadrenocortical (HPA) axis. As a result, increased levels of circulating glucocorticoids (cortisol and corticosterone) can be observed, being the main markers of stress (
31, 32). McCabe et al. reported that anxiety can be reduced with a sustained release of high dose vitamin C by alleviating the increased blood pressure as a response to stress (
33). In addition, one of the primary hormones that define stress conditions is certainly cortisol. It is known that it increases during stress conditions (
34).
In the same way, the results of our study showed that the cortisol levels were significantly higher in the group of animals treated with AW on the 21st day after treatment (p<0.001). Also, co-treatment of animals with AW and SA resulted in significantly increased cortisol levels on the 21st day (p<0.0001), showing a synergistic effect of these treatments. For reducing the stress and especially its negative effects, vitamin C or AW treatments are effective (
1, 35). Our study shows that treatment of animals with both AW and SA significantly increased the levels of cortisol on the 21st day (p<0.05), as compared to the group of animals treated with AW.
In conclusion, accumulating evidence has shown that AW and vitamin C are beneficial to the health. They suppress oxidative stress related diseases (diabetes, cancer, atherosclerosis) and neurodegenerative diseases. The essential part of the stress response is the increased production and secretion of vitamin C. The increasing cortisol production could be explained as compensating mechanism for the ascorbic mutants (
36). High levels of cortisol are a function of vitamin C deficiency. This is an alternative hypothesis, which comes from the inverse relationship of cortisol levels with vitamin C status.
CONCLUSION
The present study showed that the individual action of AW as well as in synergism with SA did not lead to significant changes in serum glucose and cortisol activity during the absence of high ambient temperature. They were effective in increasing the glucose and cortisol level during hyperthermic stress, causing a high protective effect on oxidative damage. So, the effect of oxidative cleavage is less pronounced in rats treated with AW and SA, because they have a stronger antioxidant defense that prevents oxidative modification of all biomolecules in the body.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
This research was supported by the Faculty of Natural Sciences and Mathematics, Ss. Cyril and Methodius University in Skopje.
AUTHORS’ CONTRIBUTIONS
VA and IGJ conceived and designed this research and performed the experiments. SB, VR and VA carried out data analysis. VA wrote the manuscript. VA and MA supervised the project. VA and IGJ reviewed and edited the manuscript.

 10.2478/macvetrev-2021-0023
10.2478/macvetrev-2021-0023



