The objective of our research was to estimate the therapeutic index and assess the interaction of alfaxalone (IP) with ketamine or xylazine (IM) in chicks by using isobolographic analysis. The up-and-down technique was involved to calculate the median effective anesthetic dosages (ED50) of alfaxalone, xylazine, and ketamine given separately or at the same time in young chicks. Then the up-and-down technique was involved to estimate the median lethal dosage (LD50) of alfaxalone (IP) to determine the safety profile. The ED50 of all anesthetics was evaluated isobolographically to assess the type of interaction between alfaxalone and xylazine or alfaxalone and ketamine. The alfaxalone ED50 was 32.88 mg/kg (IP), whereas the LD50 was 102.40 mg/kg (IP). The ED50 values for alfaxalone, ketamine, and xylazine were 32.88, 12.24, and 2.45 mg/kg, respectively. The ED50 values of alfaxalone with ketamine or xylazine (25:25 ED50 values) were: 7.39+2.35, and 8.61+0.63 mg/kg, respectively. ED50 values were decreased when the combinations of alfaxalone/ketamine or alfaxalone/xylazine were administered by 22-21% and 26-25%, respectively. The anesthesia of chicks with alfaxalone is safe, produces a surgical stage of anesthesia, and can be used for minor surgical procedures. The use of alfaxalone with ketamine or xylazine has been shown to have a synergistic effect and these findings may be of clinical relevance in poultry or may be extended to mammals following further clinical trials.
Surgery and painful complications like fractures, trauma, superficial corneal ulcer, and cloacal prolapse are frequently related to avian patients in veterinary hospitals. Anesthesia, therefore, plays an important role in the routine avian veterinary medicine practice. These procedures are distinct from mammalian medicine mainly because of the physiological and anatomical differences (
1). The basic cardiovascular and respiratory system anatomy and physiology are very distinct from mammals rendering chicks to be more vulnerable to anesthetics (
2). In contrast to other vertebrates, the poultry respiratory system has different ventilating mechanisms and exchange partitions (
3).
Alfaxalone is a neuroactive steroidal anesthetic with a moderately acceptable safety margin and low cardiovascular or respiratory complications (
4). It has a mechanism of action resembling propofol and barbiturates, by achieving agonist activity on gamma-aminobutyric acid (GABA) receptors, subtype-A in the central nervous system (CNS). GABA is an essential part of the CNS neurotransmitter. Alfaxalone increases GABA’s effects on its receptors by triggering ion channel opening and chloride ion inflow in the cell. This contributes to cell hyperpolarization and neuronal impulse transmission suppression. This effect is not cumulative and can be maintained by continuous rate infusion (
5). In the last years, a combination of 9 mg/ml alfaxalone and 3 mg/ml alphadalone acetate was commercially available as a neurosteroid medication (Saffan, Schering Plough Animal Health, UK). This formulation was associated with severe adverse reactions in dogs related to histamine release and anaphylaxis. The solubilizing agent cremophor, a derivative of castor oil, was reported to be responsible for this adverse effect (
6). In an effort to eradicate the side effects detected with drug carriers as Cremophor EL, Brewster et al suggested that alfaxalone and other drugs could be solubilized in safe carriers as hydroxypropyl-beta-cyclodextrin (HP-β-CD) (
7).
Ketamine is a dissociative anesthetic agent inducing dose-dependent unconsciousness and analgesia without muscle relaxation. Xylazine is an α2-agonist that has sedative, antinociceptive, and muscle relaxant effects. Both drugs have been reported to be used in single doses in avian species (
8). Their combined use for intravenous injection was reported to achieve “balanced anesthesia” compared to the separate use in single doses (
9, 10). This mixture offers quicker activation and safer recovery unlike ketamine alone (
8, 11), whereas the sedative and antinociception effects of xylazine were improved (
11).
Isobolographic analysis determines if the biological effects induced by a mixture of medications are higher, equivalent, or smaller than the expected effects of the individual components taking into consideration the dose additivity. Isobolographic analysis which is restricted to binary mixtures offers an unambiguous logical algebraic model for assessing the relationship between ternary and higher-order mixtures (
12). The objective of this research was to investigate the therapeutic safety of alfaxalone and its drug interaction with ketamine or xylazine in chicks (
Gallus gallus domesticus).
MATERIAL AND METHODS
Chicks of both sexes (N=28), age between 7 and 12 days, and body weight ranging from 70 to 110 g were used for the experiment. They were kept in 2 cages (75×100×50 cm, 25 chicks per cage) with wood shavings litter and were under constant light regime at 30–33 ºC (the ideal temperature at this age of chicks). Food and water were available ad libitum. Alfaxalone (10 mg/ml, ALFAXAN®Multidose, Jurox Pty. Ltd., Rutherford, NSW, Australia) was diluted in a 5 ml volume of distilled water and was injected intraperitoneally (IP) in a dose of 5 ml/kg. Xylazine hydrochloride (2%, Xylo, Holland) and Ketamine hydrochloride (10% injectable solution, DOPHARMA Netherland) were diluted in a 5 ml volume of distilled water and were injected intramuscularly (IM) in a dose of 5 ml/kg. This study was approved by the Specialist Science Committee of the University of Mosul, College of Veterinary Medicine (approval number UM.VET.2021.1).
ExperimentsEstimation of the anesthetic ED50 of alfaxalone in chicksThe alfaxalone ED
50 was estimated with the up-and-down method (
13). The chicks were monitored for anesthesia onset (losing the righting reflex and unconsciousness) within two hours. The doses of alfaxalone were decreased or increased by 10 mg depending on the anesthetic effect (
14, 15). The ED
50 value was calculated as follows:
ED
50 value = xf + K d
Where: xf = the last dose injected
K = Table’s value from Dixon, 1980 (depend on X and O symbols obtained)
d = increase or decrease in the dosage
Ataxia, relaxation, closed eyelids, loss of righting reflex, and sleep within 1-2 minutes were considered as positive signs of anesthesia induced by alfaxalone (
16).
Estimation of LD50 of alfaxalone in chicksThe up-and-down method was used for the estimation of alfaxalone LD
50 (
13). The chicks were monitored for 24 hours for the onset of toxic effects with an initial dose of 80 mg/kg alfaxalone. The dose was lowered or increased by 20 mg until the onset of death. The same method of estimation was used for ED
50. The signs of toxicity were recumbency, tachycardia, and tachypnea, followed by apnea and death within 20-30 minutes.
Drug safety of alfaxalone estimated by safety indicesThe estimated ED
50 and LD
50 values of alfaxalone were used for the interpretation and extrapolation of the drug safety of alfaxalone by using the following equations: TI = LD
50 \ ED
50 (higher TI values are correlated with higher drug safety); SSM = (LD
1 \ ED
99 – 1) × 100 (LD
1 is lowest lethal dose in 1% of the experimental sample and ED
99 is the minimum dose wanted to create the therapeutic effect in 99% of the experimental sample; higher SSM values are correlated with higher drug safety); The therapeutic ratio (TR) is the ratio of the lethal dose-25 (LD
25) and the effective dose-75 (ED
75); TR=LD
25/ED
75 where LD
25 is the dose that is lethal for 25% of the animals and ED
75 is the dose that is effective for 75% of the animals (
17). TR was used as a superior index of drug safety because it includes the curve sharpness.
Isobolographic analysisThe estimated ED
50 of alfaxalone, ketamine, and xylazine, as well as the ED
50 of alfaxalone/ketamine and alfaxalone/xylazine (25:25 of their individual anesthetic ED
50 values), were used for the isobolographic analysis which determined the level of drug interaction. The induction of anesthesia was confirmed by loss of righting reflex and consciousness within 30 minutes (
15, 18). The ED
50 value of alfaxalone was marked on the x-axis and those of ketamine and xylazine on the y-axis, connected by a straight diagonal line. The ED
50 values of the combined treatments on, above, or beneath the diagonal line were considered as an additive, antagonistic, or synergistic effect, respectively (
12, 19, 20, 21). The interaction index (Y) was estimated with the following equation da/Da+db/Db (
20), where ‘Da’ and ‘Db’ represent the anesthetic ED
50 of alfaxalone, and ketamine or xylazine respectively, while da and db are the ED
50 of alfaxalone/ketamine or alfaxalone/xylazine. The values of the Y-index were interpreted as follows: Y=1 - additive, Y<1 - synergistic, and Y>1 antagonistic effect (
15, 19).
RESULTS
Estimation of the anesthetic ED50 of alfaxalone in chicksThe ED
50 of alfaxalone was 32.88 mg/kg IP (
16).
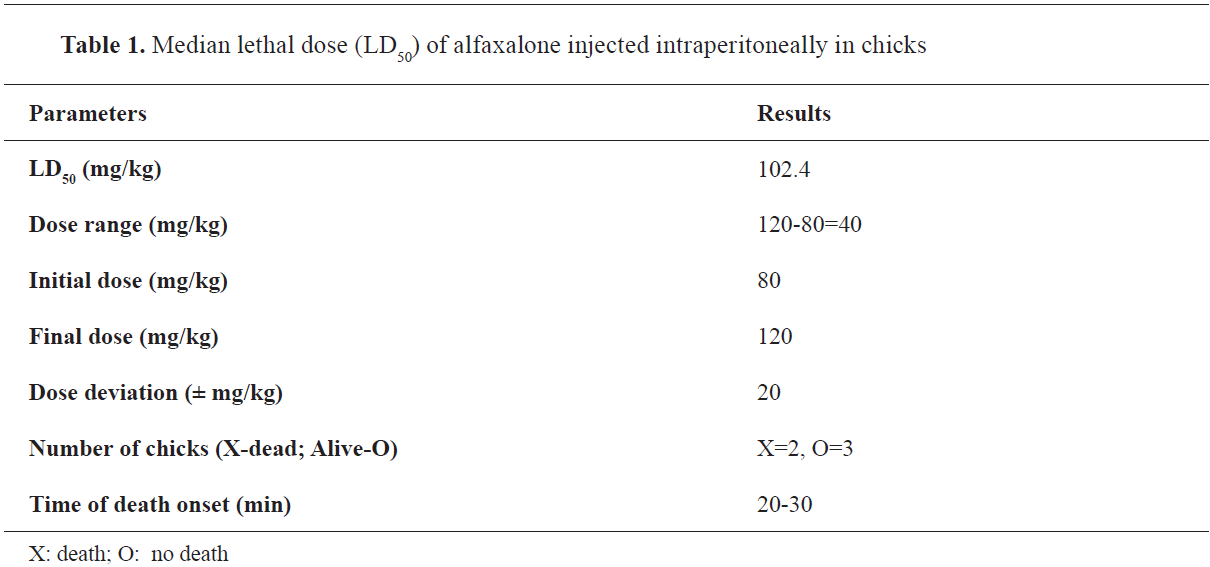
Estimation of acute LD50 of alfaxalone in chicksThe LD
50 of alfaxalone was 102.4 mg/kg IP (
Table 1).
 Drug safety of alfaxalone estimated by safety indices
Drug safety of alfaxalone estimated by safety indicesAccording to the ED
50 and LD
50 values of alfaxalone, the TI was estimated 3.1. The TI number means that the effective dose (ED
50) used of alfaxalone should be multiplied 3 times to induce the toxic effect of alfaxalone and kill 50% of the animals (LD
50) (
Table 2).
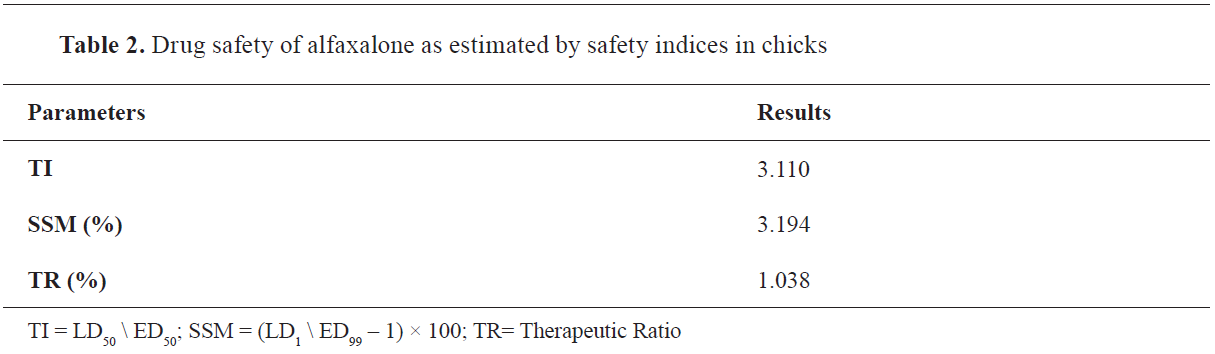
In contrast, the SSM value was 3.194%. The SSM calculated (100%) means that the effective dose of alfaxalone (ED
99) that produces 99% anesthesia in animals should be increased by 100% (i.e., from 8.514 to 17.028 mg/kg, IM) to kill 1% of the animals (LD
1) and produces its toxic and deleterious effects. Thus, the TI and SSM values reflect that alfaxalone possesses a preferable wide margin of safety. Therapeutic Ratio (TR) was 1.038.
Isobolographic analysisThe anesthetic ED
50 values for alfaxalone, ketamine, and xylazine were 32.88, 12.24 and 2.45 mg/kg, respectively. Coadministrations of alfaxalone/ketamine or alfaxalone/xylazine had an additive effect on ED
50 values 7.39+2.35 and 8.61+0.63 mg/kg, respectively. The ED
50 of alfaxalone/ketamine and alfaxalone/xylazine declined by 22-21% and 26-25%, respectively (
Table 3). Isobolographic analysis of ED
50 of the 3 anesthetics (either separately or combined) showed synergistic effects between alfaxalone/ketamine or alfaxalone/xylazine in the chicks (
Fig. 1) and (
Fig. 2). The synergistic interaction was determined by the location of the ED
50 points of alfaxalone/ketamine and alfaxalone/xylazine beneath the diagonal line that joins their individual anesthetic ED
50 values (
Fig. 1 and
Fig. 2).
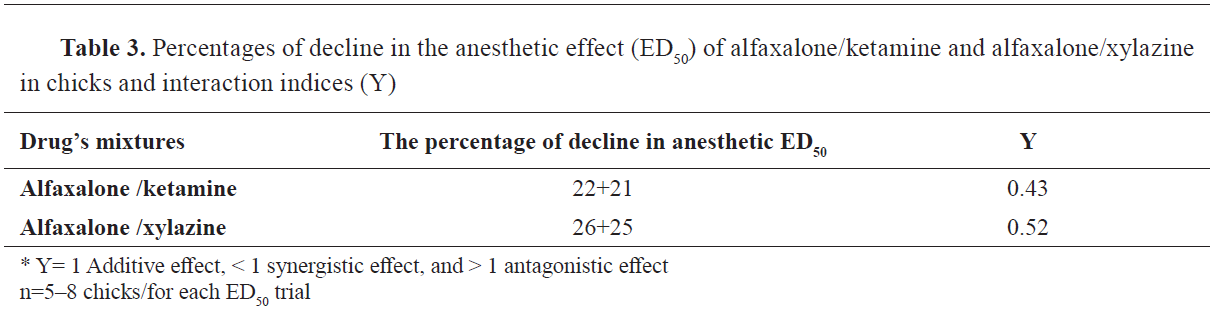
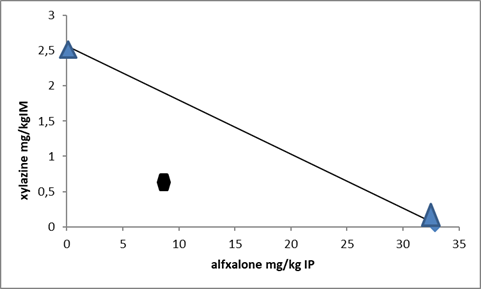 Figure 1.
Figure 1. Isobolographic analysis of the anesthetic interaction of alfaxalone and xylazine in the chicks. Points on x- and y-axes characterize median anesthetic doses (ED
50s, mg/kg) administered separately, while the circular point characterizes 25:25 of ED
50 co-administration of both anesthetics. The diagonal line between the individual ED
50s of alfaxalone and xylazine is synergism and the circular below the line refers to synergistic interaction. n=5 chicks/trial
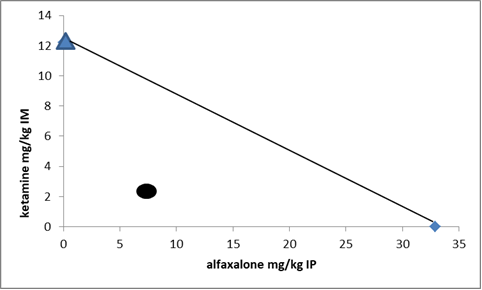 Figure 2.
Figure 2. Isobolographic analysis of the anesthetic interaction of alfaxalone and ketamine in the chicks. Points on x- and y-axes characterize median anesthetic doses (ED
50s, mg/kg) of the anesthetics administered separately, while the circular point characterizes 25:25 of ED
50 co-administration of both anesthetics. The diagonal line between the individual ED
50s of alfaxalone and ketamine is synergism and the circular below the line refers to synergistic interaction. n=8 chicks/trial
DISCUSSION
The purpose of this research was to assess alfaxalone general anesthesia profile in chicks and to evaluate the additive effects of xylazine or ketamine by increasing the anesthetic period and the anesthetic efficacy. The anesthetic efficacy was confirmed by the overall balance of anesthesia characterized by unconsciousness, analgesia, muscle relaxation, and minimal degree of reflexes. Alfaxalone was reported to induce general anesthesia at 10 mg/kg in several wild reptile species (
22). Smith and Rodriguez-Barbon (
23) reported a dose of 5-10 mg/kg IV or IM for waterbirds, but they did not provide the exact safety margin. The use of cyclodextrin with alfaxalone is poorly reported. The combination of alfaxalonealphadalone, previously used with cremophor, was reported in several avian species. A similar finding was expected to be obtained in the current formulation. The alfaxalone/alfadolone dose for budgerigar was reported 16 and 156 mg/kg IM or IP, respectively (
24). Hence, the route of drug administration is a crucial factor for the anesthetic dose. Due to the difficulty of IV administration of alfaxalone in chicks, we found that the anesthesia induction dose is larger than the IV-injected dose. According to the results of TI, SSM, and TR, we found that the alfaxalone is safe and has a good margin of safety. Due to the wide therapeutic index between ED
50 and LD
50, the alfaxalone dose could be increased threefold before initiating its toxic effects. A flatter curve is representing a higher toxic effect, hence the hyperreactive groups are at a much greater risk than the hyperactive or normal groups. Shallower curves usually have low therapeutic ratios (
17). Several studies have investigated the drug interaction between alfaxalone with xylazine (
25), ketamine (
26) in mice, rats (
27), and horses (
28).
In the current study, the anesthetic effect of alfaxalone/ketamine or alfaxalone/xylazine combinations was found to be synergetic. The isobolographic analysis showed that the anesthetics should be combined in a 0.25:0.25 ratio in respect of their individual ED50 values. The resulting interaction index was <1 (0.43 and 0.52, respectively). The synergistic effect in achieving central depression may be due to the different anesthetic mechanisms.
Synergistic effects between alfaxalone and midazolam have been described in dogs (
29) and cats (
30). It seems that the addition of ketamine and xylazine resulted in balanced and durable anesthesia for dogs, cats (
16), and mice (
25, 26). The higher anesthetic effect achieved with a low-dose alfaxalone in combination with ketamine or xylazine in chicks compared to mice could be attributed to the physiologic adaptations including a higher metabolic rate in the rodents (
31).
CONCLUSION
The result from this research indicated that the use of alfaxalone was effective and safe anesthetic for short-duration surgical procedures in chicks. Moreover, it was more efficient when combined with ketamine or xylazine by achieving balanced anesthesia. This effect was observed with a lower dose of alfaxalone combined with ketamine or xylazine. These findings may be beneficial in extrapolation of the results to the mammalian species in further research.
CONFLICT OF INTEREST
The authors declare that they have no potential conflicts of interest.
ACKNOWLEDGMENTS
The study was supported by the College of Veterinary Medicine, University of Mosul, Iraq.
AUTHORS’ CONTRIBUTIONS
ASN and AA have played a major part in designing, acquiring, analyzing, and interpreting data, performed the statistical analysis, wrote and have critically reviewed the article for essential intellectual elements, approved the final version to be issued. They were equally involved in the planning, analysis, evaluation, and writing of the article.

 10.2478/macvetrev-2021-0025
10.2478/macvetrev-2021-0025




