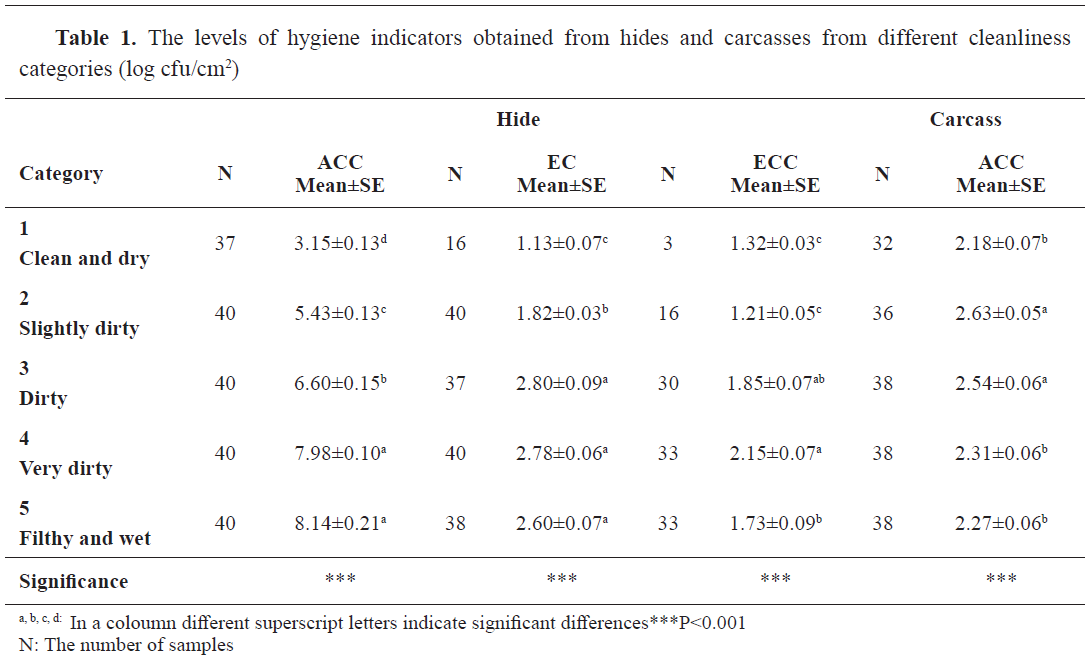
This study aimed to investigate the effects of cleanliness scoring on the microbiological load of hide and the final contamination of cattle carcasses. Fifty cattle were classified from 1 (clean and dry) to 5 (filthy and wet). Aerobic colony count (ACC) and counts of Enterobacteriaceae (EC) and E. coli (ECC) were determined on the brisket, abdominal midline, rump, groin sites of the hides, and brisket, flank, groin, and hock of the carcasses. On hides, ACC ranged from 3.15±0.13 log cfu/cm2 in category 1 to 8.14±0.21 log cfu/cm2 in category 5. EC and ECC were ranging between 1.13±0.07 and 2.80±0.09 log cfu/cm2, and 1.21±0.05 and 2.15±0.07 log cfu/cm2, respectively. While the mean ACC on the carcasses ranged between 2.18±0.07 and 2.63±0.05 log cfu/cm2 irrespective of the categories, Enterobacteriaceae and E. coli could not be counted due to the detection limits. It was concluded that although the level of bacterial load increased significantly (P<0.001) with the increasing cleanliness category on the hide of the animals, the reflection of this increasing trend on carcasses and different parts of the carcasses were inconsistent and the hygiene provided in the slaughterhouse and processing line was the main factor to reduce cross-contamination during processing.
It has been generally accepted that there are some interactions between the cleanliness of animals before slaughter and the level of dirt and filth (mud, bedding, manure, etc.) transferred to the carcasses. Appropriate measures on the slaughtering line, especially during the dressing procedures, may limit the cross-contamination from hides to meat via knives, hands, etc. However, published reports on that matter are rather conflicting to several extents. According to some studies, cattle hide plays an important role in the microbial cross-contamination of beef carcass meat during the skinning operation (
6, 7). In contrast, Van Donkersgoed et al. (
8) reported that there was no consistent connection between tags (mud, bedding, and manure) attached to the skin of cattle at slaughter and bacterial load of carcasses. Changes observed in bacterial counts, associated with several parameters such as tag scores, hide wetness, line speed, or tag shaven off the hides, were lower than 0.5 log cfu/cm
2. Antic et al. (
9) also did not find any significant differences in microbial levels between bovines with different visual hide cleanliness scores. Buncic et al. (
10) reported that the correlation between cattle hide cleanliness and hide microbiological status before and after slaughtering was limited and inconsistent, and should be further investigated.
Visually, dirtier hides are generally associated with the significantly higher aerobic colony (ACC) and
Enterobacteriaceae count (EC) on hides and dressed carcasses (
11). Food business establishments, including slaughterhouses, are required to apply good hygienic practices (GHP) and hazard analysis and critical control point (HACCP) principles-based food safety procedures (
12, 13). The regulation (
12) emphasizes that food business establishments rearing animals should ensure the cleanliness of animals during production and/or slaughter. Similarly, EC Regulation 853/2004 (H2) (
13) states hygiene control requirements for slaughter and emphasizes that all animals should be ‘clean’ before being accepted by the slaughterhouse. In several countries, such as France, Belgium, and the United Kingdom, the cleanliness of animals has been defined as an evaluation criterion. Excessively dirty animals should not be accepted for slaughter (
6, 14, 15, 16).
Despite the existing legislations in Turkey enforced by the official veterinarians which require that animals should be clean before slaughter, there is no practical application of carcass cleanliness scores (from 1 to 5) at the abattoir level (
14, 17).
The work presented here aimed to determine the effect of visual cleanliness of cattle hide on carcass contamination as indicated by ACC, EC, and
E. coli count (ECC) in cattle slaughtered in an abattoir in the Western part of Turkey.
MATERIAL AND METHODS
Cleanliness category evaluation and samplingAll visual inspection and slaughtering procedures of this work were carried out in a middle-scale slaughterhouse located in the Western part of Turkey. The slaughterhouse belonged to the municipal government of that region with a high level of hygienic measures, complying with all the requirements of the Turkish Food Safety Authority, and controlled by both municipal and state veterinarians. The premises had effectively employed the HACCP plan and GHP applications. Visual inspection procedures (scoring) were conducted according to the Food Standard Agency Guidelines (
14) while animals were at the lairage. All cattle categorized were male Holstein-Friesian calves, received from various farms. The procedure applied was simply categorization of cattle from 1 (clean and dry) to 5 (filthy and wet) based on their cleanliness. Fifty cattle (10 animals for each cleanliness category) were selected for sampling after visual scoring. Sampling was conducted immediately after bleeding when the animals were hanged by their feet. Both scoring and sampling procedures were conducted on 5 different days.
Before the slaughtering procedure, just before the trail started, the knives, and steels were immersed into alcohol (90% ethanol), were flamed and placed into holding rocks with circulating water at 82-85 °C to prevent contamination. Manipulations conducted during slaughtering procedures (e.g., skinning) for each carcass was carried out using 2 knives which were sanitized in hot water lines before their next use and/or whenever it was necessary.
Contamination was avoided by eliminating contact with aprons, water splashing, and gastrointestinal rupturing during evisceration. Samples from the hides were taken immediately after bleeding and before hide removal from brisket, abdominal midline, rump and groin sites of the animals, where most of the manipulations and handling are considered to be carried out during hide removal. At the end of the splitting procedure and just before chilling, carcass samples were collected from each carcass’s brisket, flank, groin, and hock (
6).
Microbiological analysisThe microbiological results were evaluated based on the colony counting method (18), expressed in cm
2 on swabbing areas of 25 cm
2. For each cleanliness category, 40 samples were taken from the hides and carcasses, which were assessed for ACC, EC, and ECC.
Both hide and carcass were sampled by using the double swabbing method in which swabs (Or-Bak Swap 150502) wetted with buffered peptone water (Oxoid CM 1049) were rolled on the surface of hide/carcass by using a template of 25 cm
2 surface area. Following this, dry swabs were rolled over the same area. Both swabs (dry and wet) were put into tubes containing 10 ml of buffered peptone water (Oxoid CM 1049). Samples were transferred within 1 hour in iceboxes (4±1 °C) containing cold packs to the microbiology laboratory of FVMADU, Department of Food Science and Technology.
The ACC levels and the numbers of
Enterobacteriaceae and
E. coli were determined in the samples of hides and carcasses. The tubes containing swabs were stirred by using a stirrer (Biosan, EU) for 2 min and serial dilutions were made. The inoculations from the dilutions were applied on Plate Count Agar (Oxoid CM 463), Violet Red Bile Glucose Agar (Oxoid CM 485), and Tryptone Bile X-Glucuronide Agar (Oxoid CM 945) for ACC and enumeration of EC and ECC. The plates incubation was performed at 30 °C for 48-72 h for ACC and 37 °C for 24 h for EC (
19, 20). For enumeration of ECC, Tryptone Bile X-Glucuronide Agar plates were incubated at 37 °C for 4 h and were then moved to another incubator at 44 °C for 18-24 h. Following incubation, opaque colonies with bluegreen color were identified as
E. coli (
21).
Statistical analysisSPSS version 22 (USA, 2013) was used for statistical analysis. The significances between the cleanliness categories for both carcasses and hides (P<0.05) were evaluated by using Kruskal-Wallis Variance Analysis for ACC, EC, and ECC on hide and only for ACC on the carcass. Groups with significant differences (P<0.05), were further analyzed with the post-hock Duncan test.
RESULTS
The results obtained from hide samples showed that ACC, EC, ECC, and the numbers of countable plates had an increasing trend with each consecutive category. In Category 1 there were 16 positive countable samples (40%) for EC and 3 (7.5%) for ECC.
ACC ranged from 3.15±0.13 log cfu/cm
2 in Category 1 to 8.14±0.21 log cfu/cm
2 in Category 5 (
Table 1). There were significant differences between the categories (P<0.001), except between Category 4 and Category 5. The levels of EC on the hide samples and the numbers of countable plates increased with the increasing categories, but this trend was not consistent for all groups as seen for Category 1, 2, and 3 (Table 1). No significant differences were found between categories 3, 4, and 5. The mean EC level was 1.13±0.07 log cfu/cm
2 for Category 1, but it was 2.60±0.07 for Category 5. The ECC levels had the lowest mean value in Category 2 (1.21±0.05 log cfu/cm
2) and the highest in Category 4 (2.15±0.07 log cfu/cm
2) (P<0.001) (
Table 1).
In carcass samples, results of ACC were ranging between 2.18±0.07 and 2.63±0.05 log cfu/cm
2. The lowest value was observed in Category 1, whereas the highest was observed in Category 2. The elevation observed in ACC was not consistent with the cleanliness scores. There were also significant differences between the categories, however, they were also inconsistent with the cleanliness scores (
Table 1). Data of EC and ECC could not be analyzed due to the detection limit of the microbiological examinations which was 0.36 log cfu/cm
2.
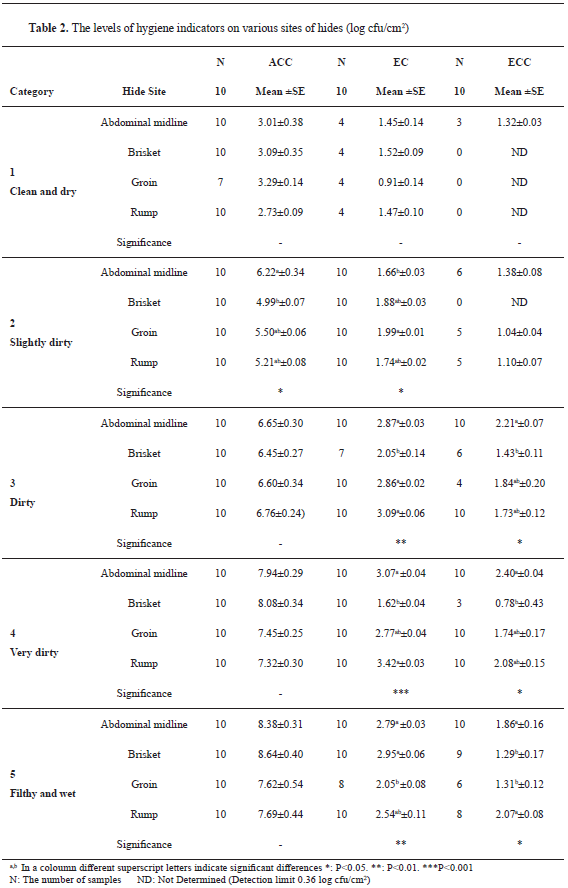
The results of the samples taken from various parts of hides did not show any significant differences for ACC except in Category 2 (
Table 2). In this group, there was a significant difference between abdominal midline and brisket (P<0.05). No differences were found between other groups (P>0.05). Despite the significant differences between sampling sites for EC (P<0.05, **:P<0.01, ***:P<0.001), the increase in the microbial counts for sampling sites obtained from different categories was not consistent. The level of ECC from different parts of hides increased with increasing category level, but the significant differences (P<0.05) between parts related to categories were inconsistent. The increase in the ECC was not relevant to the categories either.
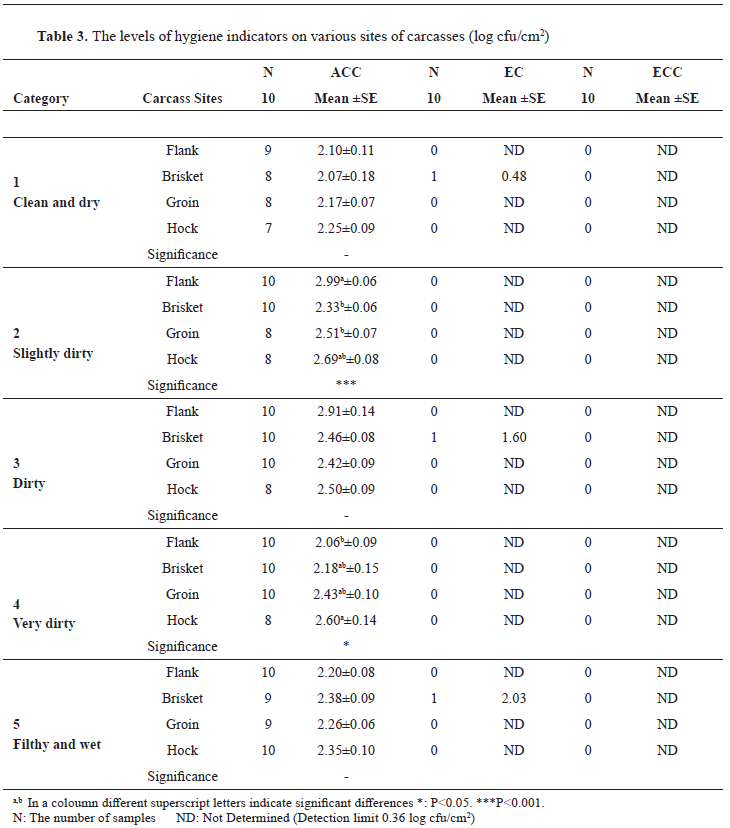
The ACC levels on different parts of the examination sites varied. Only in 2 categories (Category 2 and Category 4), there were significant differences between the examined parts. In Category 2 the highest value was on the flank area (2.99 log cfu/cm
2) whereas the lowest was on the brisket area with a level of 2.33 log cfu/cm
2 (P<0.001). For Category 4, the lowest ACC was observed on the flank (2.06 log cfu/cm
2) and the highest value was found on the hock site (2.60 log cfu/cm
2). For EC levels, plates from only 3 samples in different categories showed bacterial growth and yielded positive microbiological findings (
Table 3). For ECC no bacterial growth was determined on the plates inoculated from the neat dilution of the samples due to the detection limit of the microbiological examinations. Therefore, data of EC and ECC could not be statistically analyzed.
DISCUSSION
Animals may carry a large number of different microbiota in their gastrointestinal tract and on their hide when they reach the slaughterhouse. Hair and feet are contaminated with fecal material, especially when they are raised in intensive breeding systems. The main source of carcass contamination within the slaughter line is the feces which is in contact with the hides of the slaughtered animals scored with low cleanliness (
7). Therefore, the assessment of
E. coli and
Enterobacteriaceae’s presence on the carcass, which are considered as the most reliable indicators for contamination, can be used to evaluate the hygienic status during the slaughtering process (
22). ACC evaluation provides information concerning the shelf life (
23). However, the carcass indicators ACC and EC reflect the hygienic level, but they cannot estimate the possibility for occurrence of hazards, per se (24).
The current study showed that a higher cleanliness level (from 1 to 5) in cattle was associated with higher ACC on hides (
Table 1). This was in agreement with several other studies showing a consistent and significant increasing trend in the ACC obtained from the hides categorized according to the cleanliness scores (
6, 25). The observed increasing trend of EC and ECC levels on the hides along with the higher cleanliness categories was almost insignificant and inconsistent. Johanson et al. (
26) and Serranio et al. (
6) stated that hide portions in direct contact with soil/bedding (brisket, abdominal midline) were the most contaminated sites in all cleanliness categories. The results presented in this study were not concurring with other reports which noted differences between the parts in the same categories, which in most cases were insignificant, inconsistent, or nonexistent. Only for EC in Category 5 (filthy and wet animals), brisket and abdominal midline were heavily contaminated compared to other parts on hides.
Cattle hides generally have high bacterial loads, thus were indicated as the major source for carcass contamination (
27, 28). It was stated by several researchers that contamination mainly occurs during the de-hiding process and bacterial counts obtained from carcasses after de-hiding are correlated with those on hides (
9, 29). But, others (
8, 30, 31) reported a limited effect of hide dirtiness on carcass contamination. Reneau et al. (
32) and Ruud et al. (
33) stated that the presentation of clean cattle for slaughter and good slaughter hygiene were fundamental prerequisites for reducing carcass contamination. Buncic et al. (
10) observed that results from studies on hide cleanliness, the microbiological status of hides and carcasses were inconsistent, and further studies were necessary. Hauge et al. (
25) stated that the dirtiest animals were not associated with significantly higher carcass contamination than the cleanest carcasses, either at the start or the end of the slaughter line. A study conducted by Jericho et al. (
30) showed that there was only a weak correlation between the mean numbers of aerobes recovered from corresponding sites on groups of carcasses and the total score for visible contamination at each group of corresponding sites. Although the level of bacterial contamination increases with the consecutively higher cleanliness categories on the hide of the animals, the reflection of this increasing trend on carcasses and different parts of the carcasses are not consistent, which is in agreement with other reports (
8, 25, 30).
The contamination rate and level of indicators on the carcasses might be affected by the hygiene status of the slaughterhouse and slaughtering procedures. Ridell and Korkeala (
34) reported that contamination of carcasses from the dirty sites of the hides was highly correlated with the skills of the operator and the employed manual skinning methods. The operators’ hands might also be the source of contamination from hide / fleece to carcasses. Nastasijevic et al. (
24) reported that cross-contamination at the de-hiding and evisceration stages can be achieved by efficient trimming and washing procedures, routine sanitization of knives, equipment, and food contact surfaces, maintaining control of process hygiene, and effective monitorization of CCPs. Employment of Good Manufacturing Practices (GMPs) and Standard Sanitation Operating Procedures (SSOPs) (compliance with GHP procedures) in hygienic carcass hide removal, would additionally aid in minimizing cross-contamination (
35). The slaughterhouse which was used for sample collection for the current study had an effective HACCP procedure, GMP ongoing applications, and strict monitoring procedures. It was observed that the hygienic procedures applied in the slaughterhouse had very efficient and strict control of the workers and great attention was paid to avoid unnecessary contact between carcasses and dirty hands, knives, aprons, equipment. The premise utilized a twoknife system with a momentary dipping of the knife in hot water (82-85 °C) circulating on knife-holding rocks on the slaughtering line with fixed worker positions. Therefore, each knife was sanitized after its use on a single carcass and/or whenever it was necessary. The operators were working on either slaughtering, dressing, or eviscerating positions without rotations which might also have had some contributions to reducing the contamination rate. The automated conveyor line was working automatically ensuring minimum contact with operators during processing. These factors might have contributed to the high ACC level observed on the hide, very low level of ACC on the carcasses, and the EC level ended up with no positive carcass sample. It is generally accepted that contamination of carcasses with feces in slaughterhouses working under strict hygienic principles is very rare. It was also suggested that slaughterhouse employees especially butchers working at the de-hiding point of the slaughter line should be informed and well trained on the hygienic procedures (
9).
CONCLUSION
In conclusion, it was stated that although the cleanliness of the animal possesses great importance for the microbiological quality of carcasses, the hygiene provided in the slaughterhouse and processing line is one of the most important factors to reduce cross-contamination occurring during processing and to have low microbiological contamination on the carcasses.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
This study was supported by Aydin Adnan Menderes University Scientific Research Project Coordination (Project Number: VTF-14041).
AUTHORS’ CONTRIBUTION
EOG was included in the methodology planning, conceptualization, supervision, and writing of this manuscript. BK was included in conceptualization, analysis, resources, investigation, validation, analysis, and writing-editing of the manuscript. PK and CS performed the validation, analysis, data curation, and project administration.

 10.2478/macvetrev-2022-0012
10.2478/macvetrev-2022-0012


