The present study aimed to evaluate the changes in concentrations of some biochemical parameters, as well as macro and microscopic alterations during Eimeria stiedae infection in rabbits. The experiment was performed using 12 threemonth- old healthy rabbits, randomly allocated into 2 equal groups: G1 (controls, uninfected animals) and G2 (rabbits infected with E. stiedae). Blood samples were collected at time zero (prior to the infection), 6th, 24th, and 48th hours, and also 7th, 14th, 21st, 28th days after the infection. After sampling, the blood was centrifuged, plasma was separated and frozen at -20 ºC until analyzed. Thawed plasma was used for the quantitative determination of haptoglobin (Hp), total protein (TP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total cholesterol (TC), total bilirubin (TBIL), urea, and creatinine (CREA). The results in infected rabbits revealed a significant increase in Hp, AST, ALT, GGT, TBIL, and TC levels, as well as a significant decrease in ALP and urea. A weak hyperproteinemia was also observed. There were no changes in CREA concentration. At the end of the clinical investigation, all rabbits were humanely euthanized and necropsied. The post-mortem examination of the infected group revealed hepatomegaly, multifocal yellowish nodules diffusely spread over the liver surface and in the parenchyma, considerably dilated bile ducts, and biliary hyperplasia. Given the results obtained from this experiment, it can be affirmed that hepatic eimeriosis in rabbits is a severe parasitic disease leading to significant disturbances of liver histology and resulting changes in the biochemical profile of infected rabbits.
Rabbit eimeriosis (coccidiosis) is a highly contagious intracellular parasitic infection causedby various species of the
Eimeria genus (
1, 2, 3). The life cycle of
Eimeria spp. is complex and includes endogenous (intra-host) development (merogony and gamogony), followed by sporogony in the environment. To date, a total number of 15 valid species have been associated with rabbit eimeriosis; however, 14 are parasites of the intestines, whereas only one species (
Eimeria stiedae) infects bile duct epithelial cells and cause very severe and massive damages (
4, 5). Still, mixed infections are the most common findings, usually obtained during a routine fecal examination, although a molecular diagnosis of hepatic coccidiosis has been recently reported (
6).
Data concerning serum biochemical and protein profiles in rabbits affected by eimeriosis are mainly related to the intestinal species. The effects of treatment with toltrazuril and ivermectin for
E. stiedae infections were reported in a study thus providing information about hematological and biochemical changes (
7). Despite the potential value of Acute Phase Proteins (APP) as biomarkers of infection to monitor the health status of the animals, information about acute phase response in rabbits is limited. The APP are mainly synthesized by the liver in response to injury, inflammation, or stress (
8, 9). The concentration of APP was studied in does during lactation cycle and mastitis (
10, 11) and during
Staphylococcus aureus infection in rabbits (
12), identifying haptoglobin (Hp) as one of the major APP in rabbits. Given the above, the present study aimed to investigate serum biochemistry and Hp levels in rabbits experimentally infected with
E. stiedae along a time course of 28 days, to provide a background for early diagnosis of the disease.
MATERIAL AND METHODS
Experimental animalsThe experimental procedures were carried out according to the guidelines of the Bulgarian Food Safety Agency (Ethical Committee Approval №158/2016). A total of 12 New Zealand White healthy rabbits with an average weight of 3.2 kg, free of
Eimeria spp., were used in the experiment. The rabbits were randomly allocated into 2 equal groups: G1 (controls, uninfected animals) and G2 (rabbits infected with
E. stiedae), each consisting of six females, three-month-old animals. The rabbits from each group were reared separately into two premises without any contact with each other. During the experimental period, the animals were individually housed in metal cages with a grid floor under sanitary conditions of humidity (50-60%), temperature (15-20 ºC), and light (12 h/12 h light/ dark cycles). A two-week acclimatization period was provided before performing the experimental infection. Also, the control animals (G1) were daily examined for the presence of coccidian oocysts through routine flotation techniques to confirm the absence of
Eimeria spp. infections. Water and pellet food were supplied
ad libitum.
Preparation of E. stiedae oocysts inoculumOocysts of
E. stiedae were obtained from the livers of 2 naturally infected rabbits. After necropsy, the content of gallbladders and bile ducts was extracted by syringe and incubated with 2.5% potassium bichromate in Petri dishes at 28 ºC for 4 days. Sporulated oocysts were washed 4 times with deionized water and dialyzed against tap water for 24 hours (
13). The inoculum was concentrated to 50,000 sporulated oocysts per 1 ml. Each rabbit of the G2 group was orally infected with 1 ml of the inoculum by placing a probe.
Study design and durationBlood samples in a volume of 2 ml were collected by venipuncture of
v. auricularis caudalis from each animal into heparin tubes at time 0 (prior to the infection), 6
th, 24
th, and 48
th hours, and also 7
th, 14
th, 21
st, 28
th days post-infection of the animals (DPI). After sampling, the blood was centrifuged (2000 rpm x 10 min), plasma was separated and frozen at -20 ºC until analysis. Thawed plasma was used for determination of Hp (hemoglobin-binding colorimetric assay, Tridelta Ltd., Ireland), and other parameters as follows: total protein (TP, biuret method), total bilirubin (TBIL, modified Jendrassik-Grof assay), total cholesterol (TC, cholesterol oxidase method), alkaline phosphatase (ALP, kinetic IFCC method), alanine aminotransferase (ALT, kinetic IFCC method), aspartate aminotransferase (AST, kinetic IFCC method), gamma-glutamyl transferase (GGT, kinetic IFCC method), urea (urease method) and creatinine (CREA, Jaffe method) by using an automated spectrophotometer (Randox Daytona, Randox Laboratories Ltd), and reagents provided by the manufacturer.
Post-mortem examinationAt the end of the experiment, all rabbits were euthanized by intravenous administration of pentobarbital sodium (Repose 500 mg/ml, Le Vet Beheer B.V, The Netherlands). In order to establish gross pathology, a routine necropsy was performed following the standard protocol. For histopathological examination, liver tissue samples were collected from the lesions, fixed in 10% neutral buffered formalin (48-72 h), and embedded in paraffin. Cross-sections (4 μm thick) of the paraffin blocks (2.5x2.5x1 cm) were cut on a Leica RM 2235 microtome (Leica Microsystems, Germany) and conventionally stained with hematoxylin-eosin (H/E).
Statistical analysisThe data were analyzed using one-way ANOVA (Statistics for Windows, Stat Soft.). Obtained values were expressed as mean ± standard deviation (SD), and the differences were considered significant at P<0.05.
RESULTS
As shown in
Table 1, Hp increased in the experimental group from the 7
th DPI till the end of the study, ranging from 85.00±28.21 to 130.00±26.01 μg/ml. In contrast, a weak elevation of TP was recorded on the 14
th, 21
th, and 28
th DPI (7.70±0.75 g/L, 8.60±1.22 g/L, 8.40±1.55 g/L, respectively). The concentration of AST and ALT showed a significant increase from 7
th to 28
th DPI. The highest activity of AST and ALT was revealed on the 14
th DPI (291.0±67.0 U/L and 202.0±63.7 U/L, respectively). The GGT level was also increased between 14
th and 28
th DPI (from 26.1±17.0 to 337.0±125.0 U/L). In contrast, the ALP level decreased from the 7
th to the 28
th DPI, reaching its negative peak on the 28
th DPI (249.00±47.40 U/L). The TBIL level was increased between the 7
th and the 28
th DPI. On the contrary, the urea values in infected rabbits showed a significant decrease when compared with the control animals. Hypercholesterolemia was observed on the 7
th (126.00±41.22 mg/dL), 14
th (182.00±47.98 mg/dL), and 21
th (178.00±62.94 mg/dL) DPI in the experimental group. All additional results are summarized and presented in
Table 1.
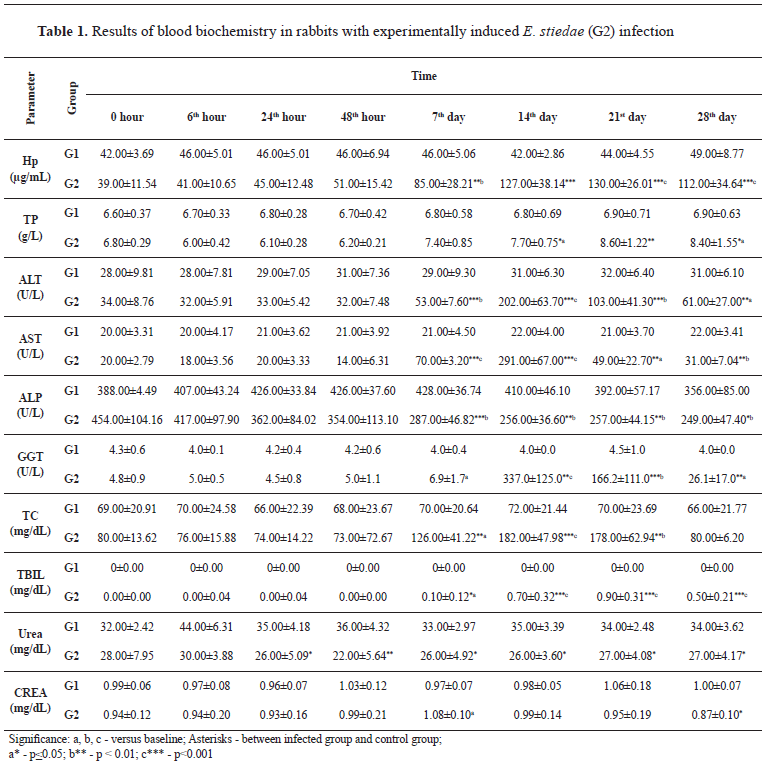
Necropsy of the corpses revealed the presence of a scant amount of yellowish transparent peritoneal fluid (about 100 ml) in the abdominal cavities; hyperemic intestinal serosae; watery content in the small and large intestines; enlarged and dark-brown colored livers (hepatomegaly and congestion) with multifocal whitish to yellowish nodules (about 1 mm in diameter) diffusely spread over the surface and in the parenchyma; considerably dilated bile ducts; distended gall bladders filled with watery yellowish bile (
Fig. 1).
 Figure 1.
Figure 1. Multifocal to coalescing yellowish nodules diffusely spread over the liver surface in rabbit with experimentally induced hepatic eimeriosis
Histopathological examination revealed multifocal foci of liver necrosis; proliferation and greatly thickened bile ducts; biliary hyperplasia, and presence of numerous
E. stiedae developmental stages (
Fig. 2).
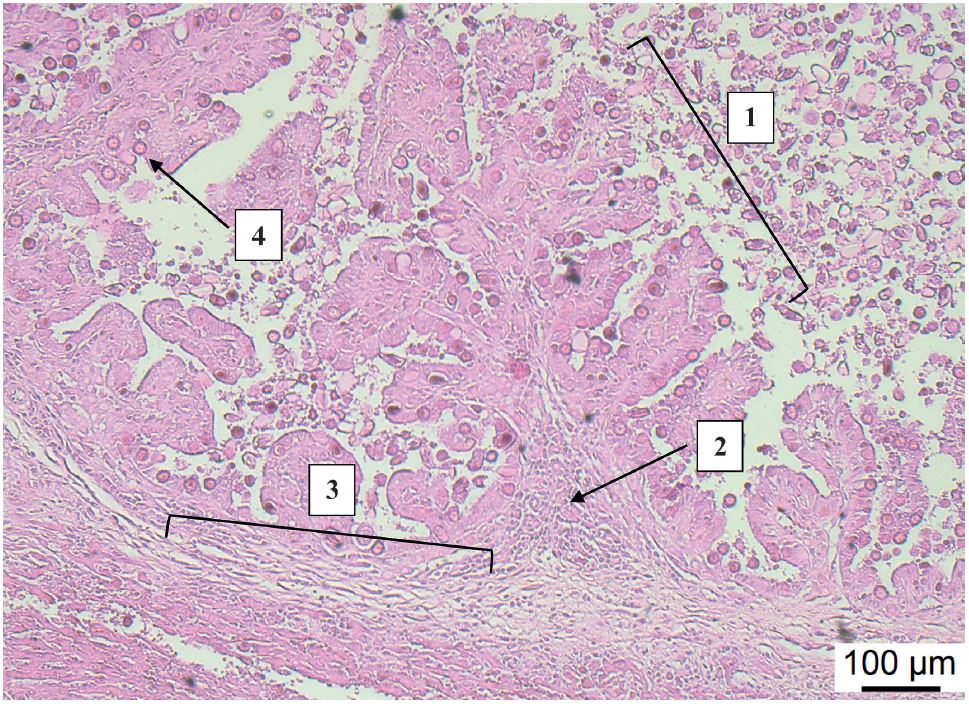 Figure 2.
Figure 2. Hyperplasia of biliary duct mucosa (1), accompanied with inflammatory round cell proliferation (2), periductal fibrosis (3) and numerous developmental stages of
E. stiedae (
4). Biliary duct section, rabbit. (H&E, Bar=100 μm)
DISCUSSION
Eimeria stiedae is one of the most important protozoan parasites affecting the rabbit liver function. This study measured several biochemical blood parameters after experimental infection with an isolated pathogen, aiming to find clues for early diagnosis, and demonstrating that several changes could be found in serum from infected animals as compared to healthy controls.
As a marker of systemic inflammation, Hp is regarded as one of the major APP. It is broadly used as a diagnostic marker in animals (
8), including rabbits (
14). The experimental challenge with
E. stiedae caused an increase in Hp levels from 7
th DPI onwards, corresponding to what has been previously reported (
15), and is likely related to the hepatic lesions resulting from
E. stiedae reproduction. The TP levels remained unchanged until 21
st DPI when a weak elevation has been observed, as already reported (
7, 16). Authors revealed that rabbits infected with either hepatic or intestinal
Eimeria species (or both) demonstrated numerous changes in blood parameters including increased levels of TP. According to Аl-Мathal (
17),
E. stiedae leads to hyperproteinemia on the 28
th DPI which was in agreement with our results.
Transaminases, GGT, and ALP are classically termed liver enzymes, but they can be found in various body tissues (
18). ALT and AST increase in the blood primarily when hepatic cytolysis occurs, whereas increased GGT and ALP activities are usually associated with cholestasis and/or biliary tract hyperplasia. In this experiment, the infection with
E. stiedae increases the concentration of ALT and AST as compared to control, indicating the presence of hepatocellular damage, as previously reported (
7, 17), and gradually decreases the concentration of ALP, which disagrees with the report of Аl-Мathal (
17). Our results do not comply with Hana et al. (
19) who revealed insignificant changes in AST and ALT in rabbits infected with
E. magna. Established differences could be a result of the topographic localization of this parasite in the intestines of rabbits. GGT level became also indicatively increased in the G2 experimental group, being likely attributed to cholestasis, as a consequence of bile ducts obstruction due to
E. stiedae reproduction.
The TBIL is a yellow pigment that may be indicative of liver function. Its abnormal rise indicates an inability of the liver to conjugate or eliminates the bilirubin properly.
Еimeria stiedae infection usually causes severe alteration of the biliary system (biliary blockage), leading to an increase in blood TBIL levels. Our results also demonstrated such a significant increase between 7
th and 28
th DPI, corresponding to what has been previously reported (
16, 17, 20, 21). The same research teams also revealed bilirubinemia in rabbits infected with
E. stiedae.
Scientific reference concerning the changes in blood TC level in rabbits infected with
E. stiedae is poorly documented. A significant increase in TC during naturally or experimentally induced hepatic coccidiosis has been reported (
16). The results of our study indicated a presence of hypercholesterolemia on the 7
th, 14
th, and 21
th DPI that seems to be in general agreement with findings of Freitas et al. (
22) showing that the most pronounced changes have appeared between the 14
th and the 28
th DPI. A reason for the increase in the TC could be due to the aggressive reproduction of
E. stiedae leading to severe morphological and functional hepatic disorders. In addition, impaired liver function is associated with deficiency of Apolipoprotein AI needed for HDL-c production that results in an increase in TC; furthermore, the low HDL-c levels hinder the reverse cholesterol transport pathway (
22). In this regard,
Eimeria bovis infection induces a parasite-related change in the host cellular sterol profile due to the vast requirements of the parasite for cholesterol during macromeront formation (
23).
The values of the urea were significantly lower from the 24
th hour to the 28
th DPI. These changes could result from the damages in the liver caused by
E. stiedae. Cam et al. (
7) have not found statistically significant changes in the urea levels which disagrees with our findings. These differences are probably due to the infective doses used in the two experiments, e.g., 50,000 oocysts of the present study as compared to 40,000 oocysts of the previous investigation (
7). The higher infective dose probably leads to more severe liver damages, resulting in a decrease in blood urea concentration. In contrast, the values of CREA remained mostly unchanged throughout the study suggesting that
E. stiedae might not affect the renal function.
Regarding the histopathological findings of rabbit hepatic coccidiosis, several authors reported similar changes, including great enlargement of the liver; multifocal to coalescing yellowish to white nodules of different sizes containing creamy fluid packed with oocysts; enlarged and distended gall bladder; proliferation of bile duct epithelium with the presence of different developmental stages of
E. stiedae; compression atrophy of the liver parenchyma around the bile ducts affected; congestion and dilation of central veins; fibrous tissue formation and lymphoid cell infiltration at the periphery of the bile ducts (
5, 7, 24, 25, 26). These findings are in general agreement with the results of the current examination.
CONCLUSION
This study investigated some serum biochemical parameters in experimentally induced hepatic coccidiosis in rabbits, presenting evidence that Hp, AST, ALT, and GGT could be useful for diagnosis but mostly in prognosis and monitoring purposes for this disease. Moreover, decreasing ALP activity is also relevant and specific for monitoring of the disease. In contrast, the creatinine level is not a specific marker for rabbit eimeriosis.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
This investigation was supported by the Faculty of Veterinary Medicine - Stara Zagora. The authors would like to thank colleagues from the Departments for the cooperation and contribution of this work.
AUTHORS’ CONTRIBUTIONS
JPP and TMG conceived and designed the study. PTI and AII prepared the infectious material and performed the laboratory animals experimental infection. IIK and NNK were responsible for the pathological study. FC, TV and AG performed the laboratory analysis of the blood samples. TMG and PTI wrote the manuscript. FC, VSP, TMG and AII revised the manuscript and approved it for publishing.

 10.2478/macvetrev-2022-0013
10.2478/macvetrev-2022-0013


