Standing and lateral recumbency positions have been used as a standard approach for most surgical procedures for large and small ruminants, respectively, without appropriate attention to the associated surgical stress. The study aimed to assess the level of surgical stress in Kano Brown goats (KBGs) based on their serum amyloid A (SAA) profiles by undergoing rumenotomy in lateral recumbency (Rumen Skin Clamp Fixation-RSCF and Stay Suture Rumenotomy-SSR) and a standing position restraint. A total of 24 KBGs were equally allocated by number and sex in groups A, B, C, and D. Groups A and B underwent RSCF and SSR in lateral recumbency, respectively, while group D underwent a rumenotomy in a standing position performed in a custom made mobile small ruminant surgical chute. Animals in groups A, B, and D were diagnosed with rumen foreign bodies, whereas group C was used as a control with a negative diagnosis. At 48 h post-rumenotomy, the mean SAA concentration in group B (137.88±66.87 g/L) was significantly higher (P<0.05) than the value in group C (34.59±0.57 g/L). The females in group B had a significantly higher (P<0.05) mean concentration of SAA at 48 hours (210.15±123.73 g/L) than groups C (35.18±0.08 g/L) and D (48.35±12.15 g/L). In group A, at 24 hours, SAA concentration (115.61±20.96 μg/L) was significantly higher (P<0.05) than that of group B (31.51±2.59 μg/L) and group C (34.86±3.21 μg/L) in males. Rumenotomy in the standing restraint position was associated with minimal surgical stress, hence it’s recommended over the conventional lateral recumbent position techniques.
Rumenotomy is a routine surgical procedure that includes incision into the rumen (
1, 2, 3) and is used for the relief of numerous conditions, especially rumen foreign body impaction (
3, 4, 5). The procedure is commonly employed for large ruminants (bovine species), but the technique is also used for small ruminants (sheep and goats) without appropriate standardization (
6). The indications for rumenotomy, preoperative patient management, various surgical techniques, and postoperative management, including complications from the procedure, have all been described, but only limited information is available for restrained animals in various positions (
6).
Stress is defined as a detectable change in homeostasis in animals or other biological systems as a result of external and/or internal vigor (
7). Surgical stress results in biochemical, physiological, cognitive, and behavioral changes that seek to either change the source of the stress or cope with its repercussions (
8). One of the many complex reactions to stressors is inflammation which involves cellular and vascular responses with the release of biochemicals such as the acute-phase haptoglobin (Hp) are positive acute phase proteins constituting the most prominent serum APPs in ruminants such as goats, sheep, and cattle. They serve as biomarkers of stress due to injury or infection (
9, 10). The intensity of the inflammatory process and the extent of tissue damage are reflected in changes in APP levels in the blood (
11, 12). Similar to SAA and Hp, C-reactive protein (CRP) is a major positive APP in humans, pigs, and dogs, but not in caprine species. In goats and other ruminants with some pathologic conditions and unclear pregnancy status, Hp must be evaluated with caution (
13). Similarly, Hp was reported to be affected among other APPs following administration of analgesics (especially NSAIDs) in goats (
14). Therefore, Hp could not be considered to be a more reliable biomarker than SAA for surgical stress assessment that would involve analgesia.
Chute or crush restraint in standing position had been the choice for large ruminant surgeries while lateral recumbent position approach is the norm for small ruminants. There is a paucity of information on attempts to mimic standing rumenotomy in small ruminants. This renders small ruminants disadvantaged to the many benefits that the chute restraint offers for the large ruminants during surgeries in standing position.
Changes in APP profiles revealed that regional anesthesia with various local anesthetic drugs and assorted suture materials caused different levels of stress response following surgery and subsequent postoperative outcomes (
7, 15, 16, 17). Hence, we hypothesized that Rumen Skin Clamp Fixation (RSCF) and Stay Suture Rumenotomy (SSR) techniques employed in standing and lateral recumbency restraint positions in the mobile small-ruminant surgical chute (MSRSC), would not elicit significantly different postsurgical stress based on the SAA profiles in male and female Kano Brown goats.
The study aimed to compare the two conventional rumenotomy techniques RSCF and SSR in a lateral recumbency position and standing restraint rumenotomy based on the SAA concentrations as biomarkers of surgical stress in Kano Brown goats.
MATERIAL AND METHODS
A total of twenty-four (n=24) Kano Brown goats (KBGs), with a body condition score of 2.95±0.34, aged 1 to 2 years, were procured for the study from a local goat market in Giwa Local Government Area of Kaduna State, Nigeria. The KBGs had a mean body weight of 15.00±0.52 kg. Foreign body impaction (RFBI) was confirmed by palpation. Eighteen (n=18) KBGs were confirmed positive while six (n=6) were negative on RFBI. The animals were accommodated in a large animal pen and were subjected to physical, hematological, and fecal examinations. They were dewormed with ivermectin (Bremamectin®, Brema pharma GmBH, Germany) in a dose of 200 μg/kg S/C, and were administered prophylactic doses of oxytetracycline (Kepro Oxytet® 20% LA in, Kepro B.V., Holland) in a 20 mg/kg IM dose. Clean drinking water was provided
ad libitum if not specified otherwise. The animals were fed adequately with groundnut husk, mixtures of beans, sorghum shafts, and maize offal, thrice daily, if not specified differently. The animals were acclimatized for two weeks before being randomly allocated into groups A, B, C, and D. Each experimental group comprised six (n=6) KBGs, three (n=3) males and three (n=3) females.
The ethical approval (No. ABUCAUC/2018/054) was obtained from the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC).
The surgical procedures were carried out at the Large Animal Surgery Theatre, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. The National Animal Production Research Institute (NAPRI) Laboratory located at the Ahmadu Bello University, Zaria, was used for conducting the laboratory phase of the study. SAA ELISA kits were procured from Abbkine Scientific (Abbkine, Inc©, China).
The animals in groups A and B were put to lateral recumbency restraint and were subjected to RSCF (
Fig. 1) and SSR (
Fig. 2).
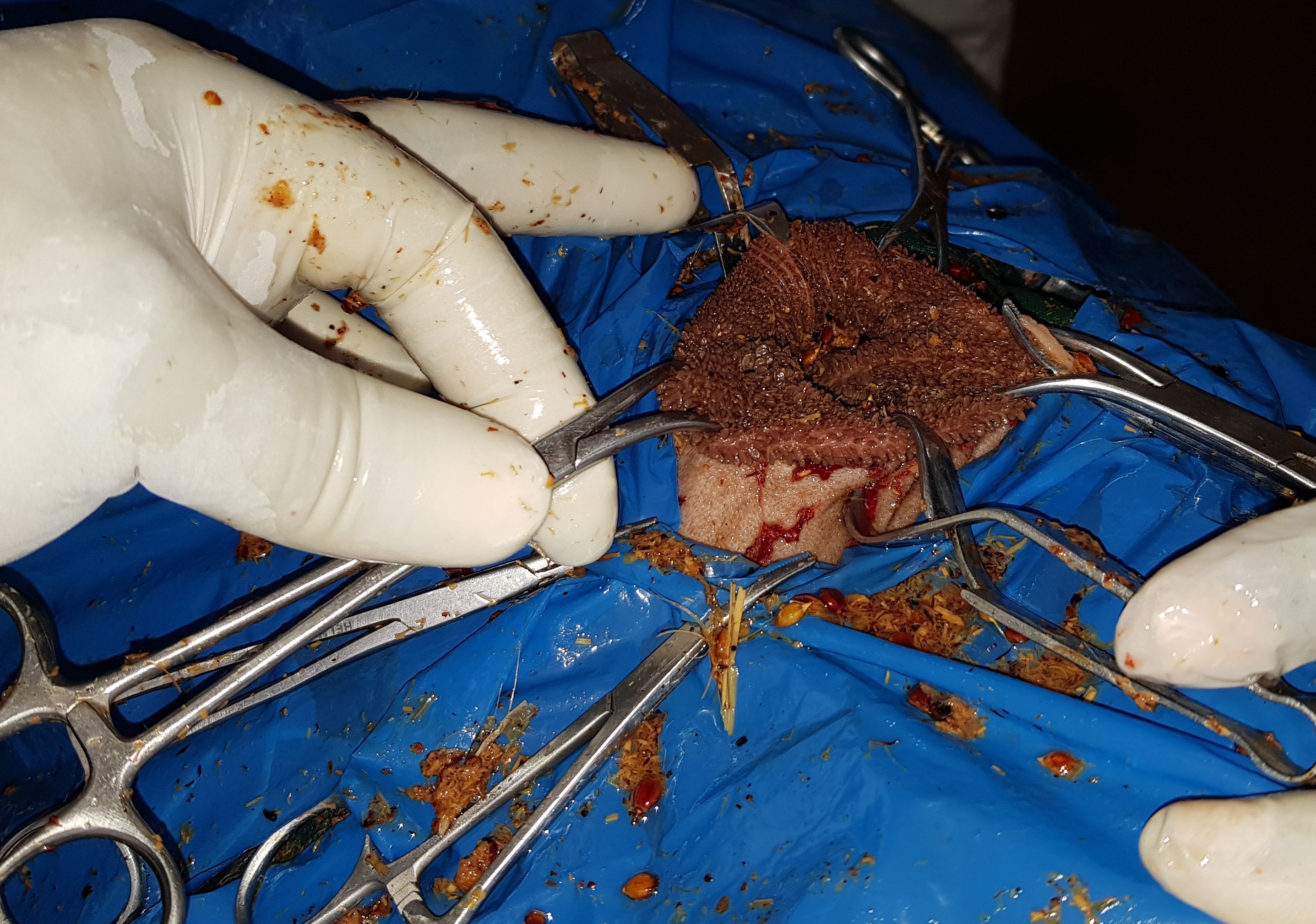 Figure 1.
Figure 1. A right lateral recumbency restraint position illustrating towel clamps securing rumen edges to the skin in a rumen skin clamp fixation (RSCF) technique of rumenotomy in Kano Brown goats
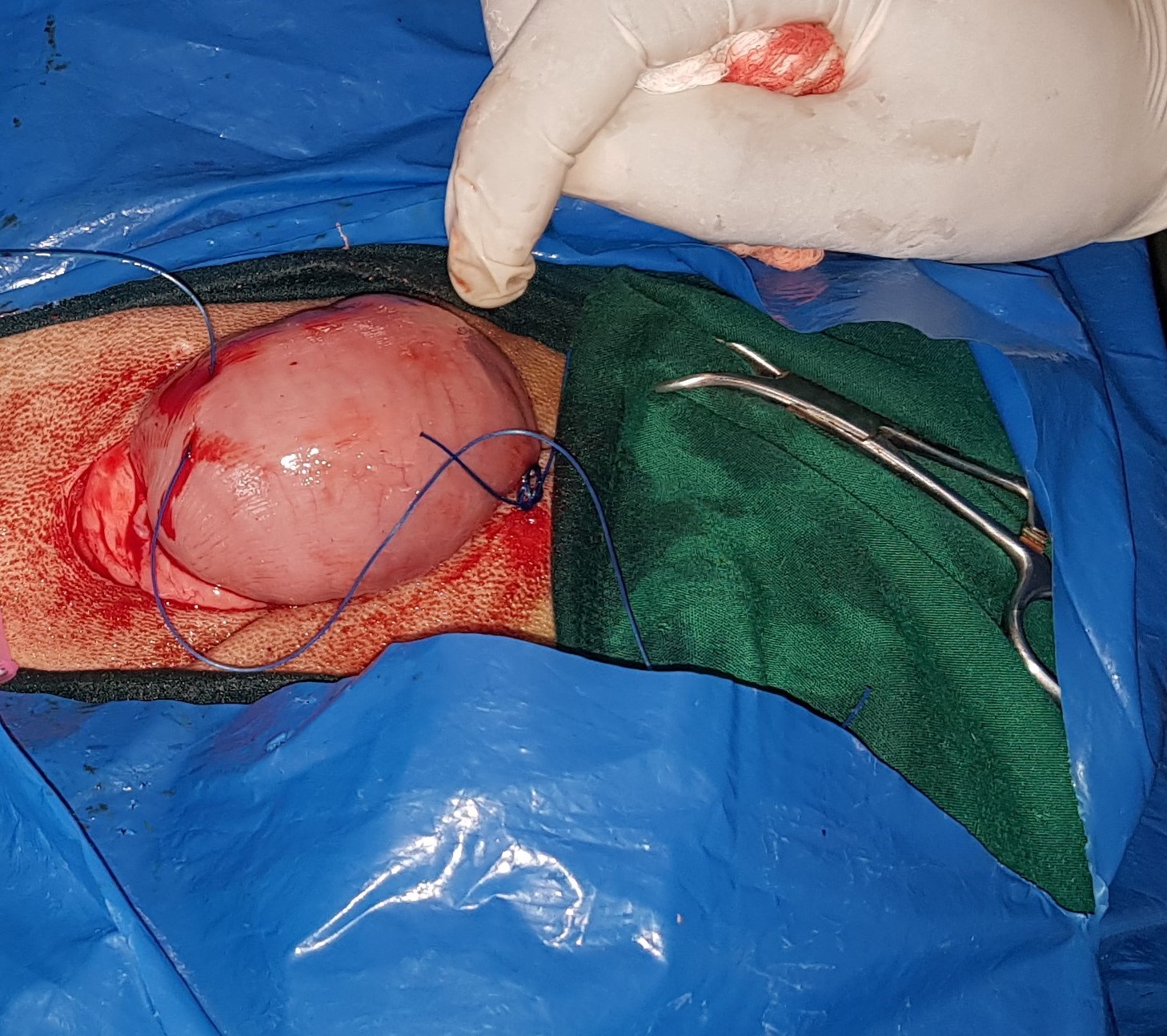 Figure 2.
Figure 2. Securing the rumen to the skin in a stay suture rumenotomy (SSR) technique following lateral recumbency restraint position
Standing restraint position was achieved by using fabricated Mobile Small Ruminant Surgical Chute (MSRSC). Group D was subjected to rumenotomy, while group C which was negative on RFBI was not subjected to rumenotomy and served as control.
Three goats were subjected to surgery on daily basis for six consecutive days following pre-surgical evaluations by 12- and 6-hours deprivation of food and water, respectively. Xylazine hydrochloride (XYL-M2® VMD NV, Belgium) was administered to all the groups in a dose of 0.025 mg/kg via a SC route which also aided in achieving standing sedation in group D. The left paralumbar fossae were shaved and aseptically scrubbed using 0.2% Chlorhexidine gluconate (Savlon®, Johnson and Johnson (Pty) Ltd, London) and povidone-iodine (Sawke-10%®, Jawa International Limited, Nigeria). Similarly, povidone iodine was applied prior to the administration of inverted L-block regional anaesthesia with 2% lidocaine HCl in a 4 mg/kg dose (NCL Lidocaine®, Syncom Formulations, NCL Pharm Chem Ind. Ltd., India). After draping and proper positioning, all animals in A, B, and D group were incised on the skin of the left mid-flank area with 7 cm incisions as described by Firth et al. (
18).
Rumen skin clamp fixation technique (RSCF)The rumen skin clamp fixation technique (RSCF) was performed after laparotomy and exteriorization of the rumen in the animals of group A. Two of the six towel clamps were used to secure the rumen dorsal sac to the skin ventrally and dorsally, towards the greater curvature. A seven-centimeter (7 cm) incision was made in the less vascularized dorsal rumen sac. A stab incision was made in the rumen wall to allow gas to escape before being extended with a sharp-blunt pair of scissors. The rumen margins were affixed 2–3 cm caudally and cranially over the skin with towel clamps, as described by Dehghani and Ghadrdani (
4). To provide abdominal cavity closure, the other clamps were used to secure the rumen borders away from the incision site. By exploration of the rumen contents, foreign bodies (mostly polythene bags) were located and removed.
Normal saline 0.9% (Juhel®, Nigeria) was spewed to cleanse the rumen ingesta before the surgical wounds were closed. To invert the rumen margins, a continuous Lambert suture pattern was used, followed by a single layer of Cushing suture pattern with a number 2 (USP) polyglycolic acid suture (Atramat®, International Farmaceutica, Planta, Mexico). Тhe peritoneum, transversus abdominis, and the internal abdominal oblique muscles were sutured as first abdominal muscle layer closure by using Chromic catgut number 2 (Lifecare®, Anhui Kangning Industrial Group Co. Ltd, China) in a simple continuous suture pattern. The external abdominal muscle and the subcutaneous tissues were sutured as a second abdominal muscle layer closure. The skin was sutured in a Ford interlocking pattern by using number 2 USP nylon suture (Lifecare®, Anhui Kangning Industrial Group Co. Ltd, China).
Stay suture rumenotomy technique (SSR)The rumen was gently wrenched out through the incision and the ruminal edges were attached to the skin with 4 nylon suture knots in a simple interrupted suture pattern (
Fig. 2).
The ruminal cavity was explored by grasping the incised edges. The closure of the incision sites was as described for the RSCF rumenotomy.
The small ruminant surgical chute rumenotomy (MSRSC)The MSRSC was applied to the animals in group D which were placed in a mobile small ruminant surgical chute (
Fig. 3).
 Figure 3.
Figure 3. A Standing restraint for rumenotomy using a mobile small ruminant surgical chute (MSRSC) with the animal’s head within a surgical guide bar (A), shaved left flank (B), laparotomy (C) and evacuation of foreign materials from the rumen (D)
The ruminal cavity was explored by grasping the incised edges. The closure of the incision sites was as described for the RSCF rumenotomy.
The small ruminant surgical chute rumenotomy (MSRSC)The MSRSC was applied to the animals in group D which were placed in a mobile small ruminant surgical chute (
Fig. 3).
The skin incision was made on the left flank (
Fig. 3) in a standing restraint position as described earlier for groups A and B. The rumen was gently towed outside and held in place by the surgeon’s non-dominant hand. Over the greater curvature of the rumen, the dorsal sac was located, and a stab incision was performed with the surgeon’s dominant hand over a less vascular portion. The incision was then extended to 7 cm with the surgeon’s dominant hand using sharp-blunt scissors and the same hand was inserted to remove all foreign bodies. The rumen was moved away from the abdominal wall during this phase to prevent contamination from the rumen ingesta. The rumen was rinsed and closed as described for groups A and B. The goats in control group C were not subjected to surgery, but they were blood sampled in the same way as the experimental groups.
In all the three groups A, B, and D, postoperative care included the provision of analgesia Inj Flunixin meglumine (Bremafluxin®, Bremer Pharma, Germany) 2.2 mg/kg 3/7 IM and antibiosis was achieved with Inj Amoxycillin trihydrate (Amoxinject®, Bremer Pharma GMBH, Germany) 8.6 mg/kg 5/7 IM. The surgical wounds were cleaned daily with chlorhexidine gluconate applied on a gauze along with a seven-day course of topical Oxytetracycline spray on the surgical sites (1/52).
Sample collectionThe blood samples (5 ml) were obtained via the jugular vein from each animal in A, B, and D group before the surgery to establish pre-rumenotomy values. Blood samples were also taken at 0, 5, 24, 48, and 72 hours after rumenotomy, as well as at weeks 1, 2, and 3. The blood samples were collected into a plain vacutainer tube and allowed to clot for two hours at room temperature before being centrifuged at 1000 g for 20 minutes to extract serum. The sera were emptied into micro-vials and stored at -20 °C for 4 weeks before the SAA ELISA analysis (Abbkine Scientific, China). The serum samples were allowed to thaw at room temperature, avoiding repeated freeze/thaw cycles.
Serum amyloid A (SAA) ELISAIn this investigation, the goat SAA ELISA kits used a two-site sandwich ELISA. The kits were produced and aimed to measure SAA in biological samples such as blood serum. A microplate had been pre-coated with antibodies specific for the goat SAA ELISA kit. Following the manufacturer’s instructions (Abbkine Scientific®), standards and samples were pipetted into the wells, ensuring that any SAA present was bound by the immobilized antibodies. After eliminating any unattached compounds, the wells were filled with HRPconjugated SAA detection antibody. A Chromogen solution was added to the wells after the wash to remove any unbound HRP reagent and to developed color in proportion to the amount of SAA bound in the initial phase. The color progression was then stopped. Using a microtiter plate reader BIO-TEK® EL800, the Optical Density (OD) was read instantly (within 15 minutes) at 450 nm. For the standards, zero standard OD was subtracted, and duplicate readings were averaged. Using the computer software Curve Expert Professional®, a standard curve was constructed by graphing the mean absorbance for each standard on the X-axis against the concentration for each standard on the Y-axis and displaying the trend line for the best fit curve through the points on the graph.
Data analysisGraphPad Prism 5.03, (2009) for Windows (GraphPad Software, USA) was used to calculate the M±SE of the variables using column statistics. To compare the four groups A, B, C, and D, a two-way repeated-measures ANOVA was employed with a Bonferroni post-test, and level of significance was set at P<0.05.
RESULTS
The mean SAA concentrations in mixed-sex, female, and male KBGs of the experimental groups are provided in
Tables 1,
2, and
3, respectively.
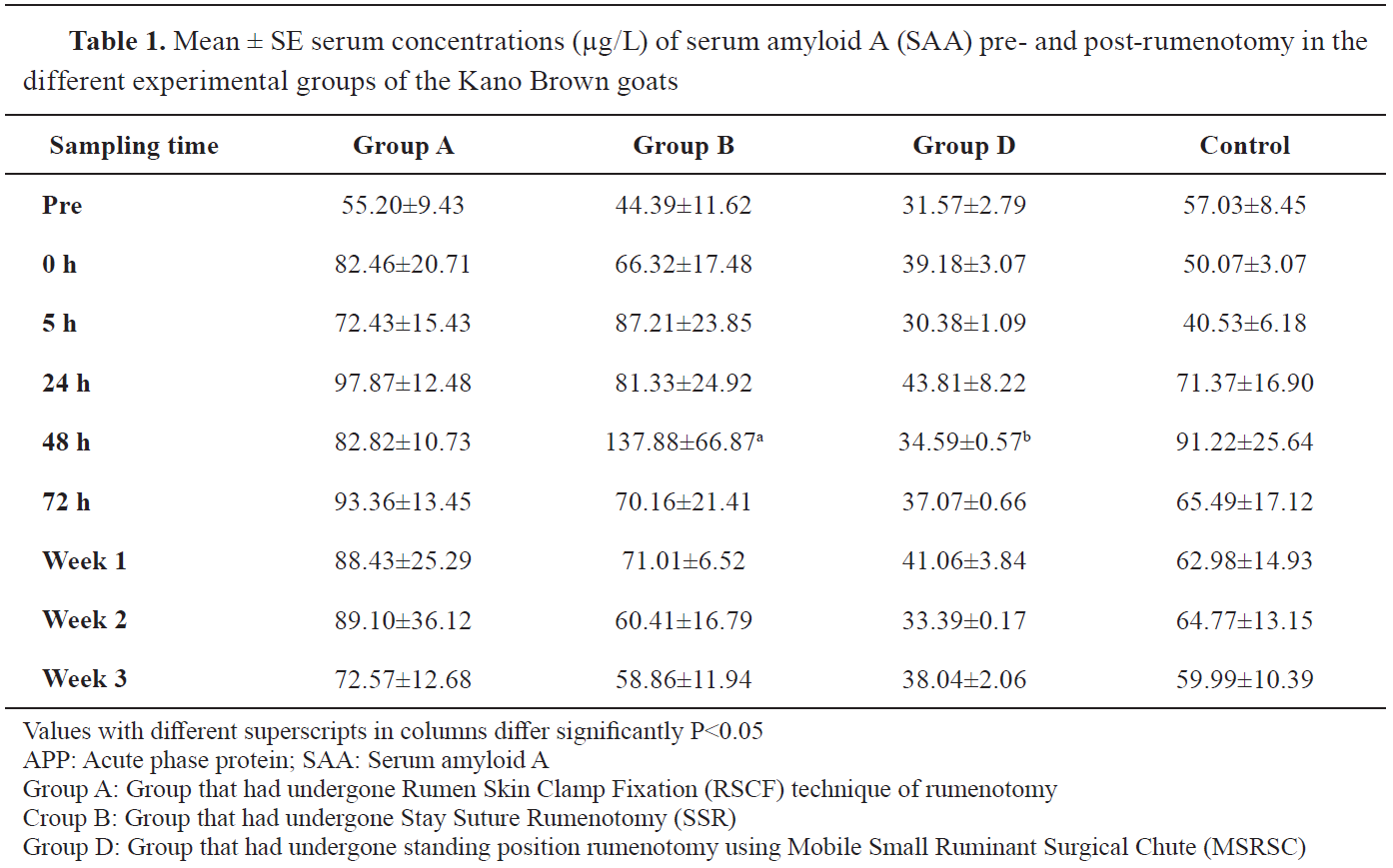
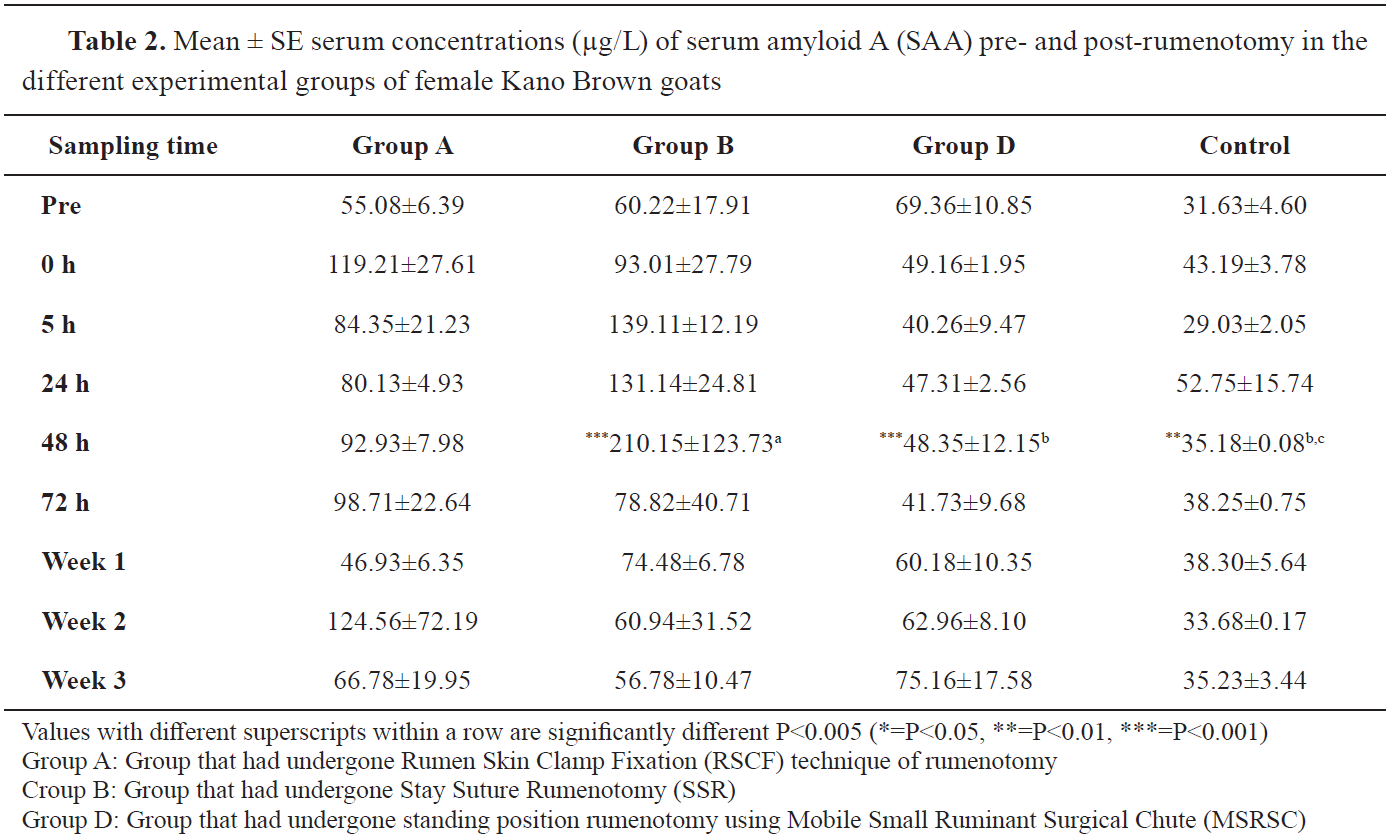
Group D had higher SAA values in prerumenotomy samples but lower at practically all intervals following rumenotomy (
Table 1). There was no significant difference between the serum concentration of SAA in group D (MSRSC) and the concentrations in the other groups. However, the mean SAA concentrations in group B (137.88±66.87 μg/L) were significantly higher than the value in group C (34.59±0.57 μg/L) at 48 hours post-rumenotomy (
Table 1).
The SAA concentrations in group D were higher than all the values in other groups at prerumenotomy but conversely, the female KBGs in group D maintained lower SAA concentrations at practically all sample intervals following rumenotomy (
Table 2). In the females of group B, the mean concentration SAA at 48 hours (210.15±123.73 μg/L) was significantly higher (P<0.05) than those of groups C (35.18±0.08 μg/L) and D (48.35±12.15 μg/L) (
Table 2).
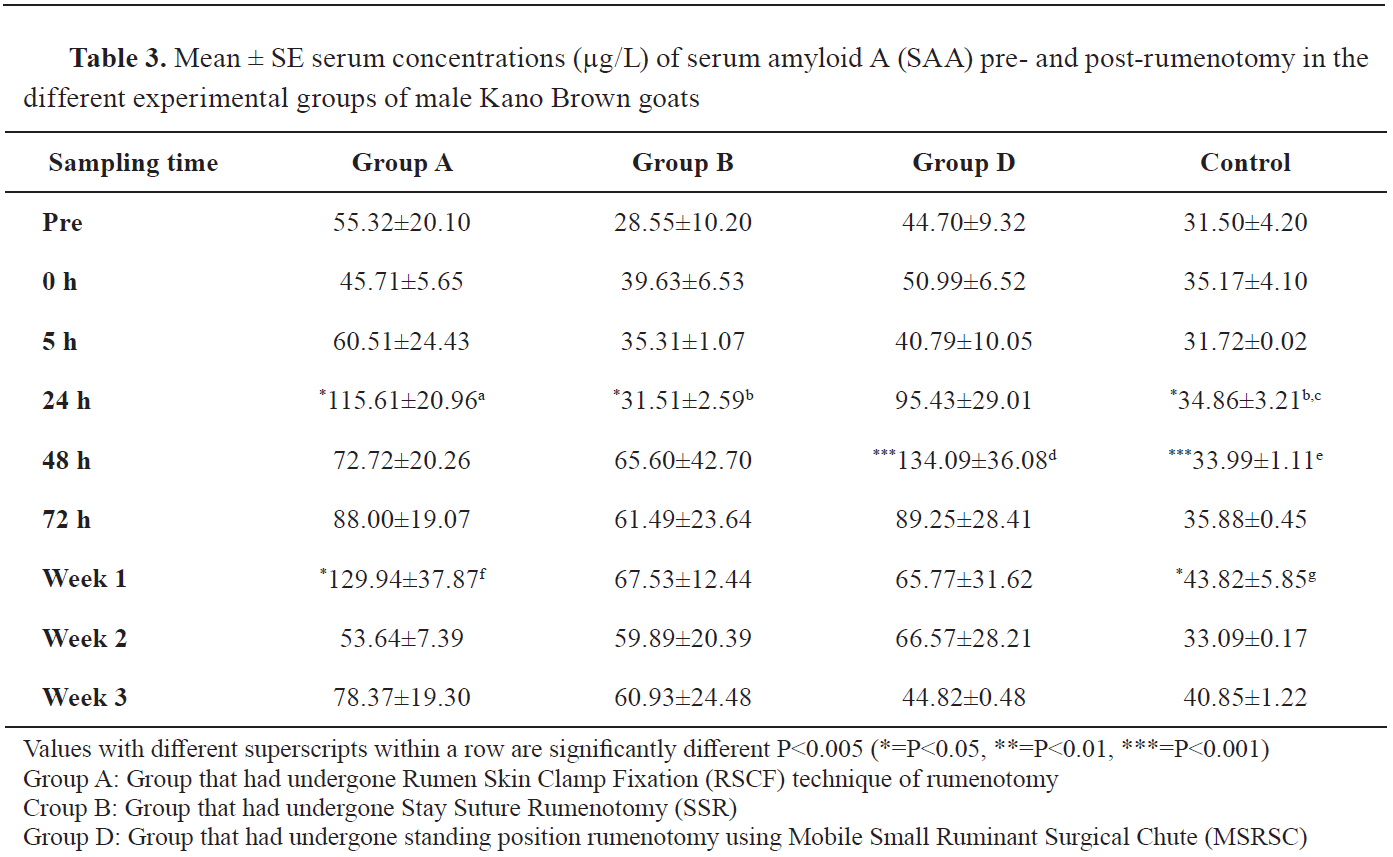
The male KBGs in group A had significantly higher SAA values at 24 hours post-rumenotomy (115.61±20.96 μg/L) compared to group B (31.51±2.59 μg/L), and group C (34.86±3.21 μg/L). No significant difference was observed between the mean SAA concentration in group D and those of the other groups in the male KBG (
Table 3).
DISCUSSION
The finding in this study that the mean serum concentration of SAA increased significantly in groups A (RSCF) and B (SSR) that had undergone rumenotomy in recumbency restraint position than in group C, suggest that the lateral recumbency position induced a more stressful effect than the standing restraint rumenotomy of the MSRSC (group D). This is because the surgical stress was positively correlated with the higher SAA response (
13, 19, 20, 21). Similarly, Saidu et al. (
16) had reported a relationship between the degree of surgical stress and the SAA level. Wen et al. (
22), also, reported an increased level of another acute-phase protein, the CRP, that was linked to the severity of surgical stress. In their study, they compared new surgical procedures in dogs as a model for human diseases. The canine model allowed them to expose the thoracic cavity and perform surgical lung biopsy via a transumbilical incision. The lower CRP indicated that this procedure could be more advantageous than the currently employed transthoracic thoracoscopy approaches.
Freeman et al. (
23), reported that several studies have compared surgical trauma by analyzing different surgical procedures in various tissues. The APPs such as the SAA are useful markers for the diagnosis and prognosis of surgical stress especially in ruminants (
12).
The female KBGs in a recumbent restraint position for rumenotomy demonstrated surgical stress at almost all the periods of evaluation of SAA than the female goats in standing restraint position. This was similarly observed in the values of SAA among the male KBGs, except for the values in group D at 48 h, which could have been influenced by an outlier value as could be suspected by its standard error. The fact that normal values of SAA are not affected by gender under normal conditions (
24), the higher levels of SAA in the female KBGs suggest their expression of more surgical stress than males in recumbent restraint and also than those subjected to rumenotomy in standing restraint position. At 24 h, 48 h and 72 h, the SAA concentrations in male KBGs from group D were higher than those in group B, however, the differences were not statistically significant. Group A, on the other hand, had greater values than group D. At 48 h, there was a substantial difference between groups D and C. However, the lack of significance between groups D and B suggests that the standing restraint position rumenotomy in an MSRSC did not significantly differ in the severity of the surgical stress compared to the lateral recumbency restraint, and thus can be used conveniently for surgeries.
These findings agree with the fact that APPs are reliable biomarkers of surgical stress especially when species-specific biomarkers are identified such as in this study (
13, 20, 25). This study also suggests that surgical stress could be associated with restraint positions for rumenotomy in KBGs based on the SAA profiles. Hence, the standing restraint induces minimal stress compared to the recumbent restraint positions for rumenotomy.
Based on their SAA concentration postrumenotomy, SSR is preferable to RSCF that underwent the same lateral recumbency restraint positions.
CONCLUSION
The MSRSC restraint for rumenotomy in the standing restraint position was associated with reduced SAA responses especially among the female KBGs, indicating that less surgical stress was instilled. Thus, for rumenotomy in the female goat employing MSRSC for standing restraint, it is advantageous over lateral recumbent restraint position, as the former substantially ease procedures. SSR is preferred to RSCF that received the same lateral recumbency restraint positions based on their varied SAA expressions after rumenotomy. Although the expression of surgical stress following standing restraint rumenotomy may not be as beneficial to the male as in the female goats, more research into the healing of rumenotomy wounds after various restraint positions and rumenotomy techniques might further provide a basis to improve the quality of rumenotomy outcomes in small ruminant practice.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The research was supported through study fellowship grant by Tertiary Education Trust Fund (TETFund) 2014/2015/2016 (Merged), University of Maiduguri, Maiduguri, Nigeria.
AUTHORS’ CONTRIBUTIONS
AMS, STF, GEO and SA participated in the design and execution of the experiment. AMS wrote the manuscript. STF, GEO and SA were involved in reviewing and editing.

 10.2478/macvetrev-2022-0014
10.2478/macvetrev-2022-0014





