Hyperthyroidism is the most frequently diagnosed endocrine disorder in cats. Therapy may include pharmacological, surgical (thyroidectomy), radioactive (iodine), and dietary treatment. The choice of treatment is believed to be strongly influenced by the veterinarian’s experience, level of education, and knowledge of the current scientific literature. The history of survival rates can affect the decision for treatment by both the veterinarian and the owner. This study aimed to explore the longevity in cats diagnosed with hyperthyroidism and to identify significant variables which affect survival rates by using retrospective data from the practice. A multivariate Cox regression was applied with the following results: surgical thyroidectomy and methimazole medication produced similar longevity (median 23.5 months, P>0.05); Domestic Short Hair cats survived longer than pure breeds (median 27.2 vs 9.4 months, P<0.05); as do cats without chronic renal disease (median 28.1 vs 6.2 months, P<0.001); and those with low activities of alanine aminotransferase (median 27.1 vs 17.0 months, P<0.01). Hyperthyroidism is comorbid with renal diseases, but no cumulative effect was found on survivability. There was no difference in survival rates between surgical and pharmaceutical treatment, therefore the discussion of treatment options with owners can focus on other factors (e.g., cost, owners’ compliance, cats’ tolerance to medication, presence of comorbidities). We propose that surgery may be the preferred treatment when the survival rates are expected to be higher than one year. This would avoid high costs and potential side effects of medication.
The treatment options include pharmacological therapy often via the use of methimazole (MM), thyroidectomy (ST), radioactive iodine therapy (
131IT) (
4), and a low iodine diet. The diet provides iodine for the production of thyroid hormone; thus, hyperthyroidism can be managed by limiting the dietary intake of iodine (e.g., feeding with complete Hill’s® Prescription Diet® y/d
TM Feline) (
5).
The experience, education, and knowledge of the current scientific literature can highly affect the veterinarian’s treatment choice (
6).
MM is one of the most commonly used drugs for the long-term treatment of HT in cats not submitted to surgery (
7). The ST is widely performed, but post-operative complications may result from the anaesthesia or by a recurrence of hypoparathyroidism and HT both in unilateral and bilateral ST (
8). In a recent study including UK veterinarians, 66% of the respondents preferred oral medication, and 28% ST (
5).
Several authors (
9, 10, 11, 12) studied survival in cats after
131IT, but there are no studies comparing survivability in cats diagnosed with HT and treated with MM or ST. Williams et al. (
13) studied survival rates in cats treated with MM or with a combination of MM and ST but did not investigate the differences between both therapies.
HT is prevalent in geriatric cats, as are several other diseases that can be comorbid and interactive with HT (e.g., heart, kidney, and liver diseases). High activities of liver enzymes can be associated with HT (
14) and oral MM drug administration can have side effects including vomiting, anorexia, and less frequently, hepatotoxicity (
8). The history of survival rates following treatment can highly affect the clinical staff and owner’s treatment choice. Higgs et al. (
6) reported that 95% of the respondents (veterinarians) stated that the owner’s compliance with the medication protocol, drug administration, and comorbid disease in patients, are important factors in formulating a management plan. However, they note that there is a lack of consensus on the impact of comorbid disease.
By using retrospective data from the practice, the study aimed to explore the survivability in cats diagnosed with HT, and to identify variables that can significantly indicate survival rates based on the treatment, breed, age, and comorbid conditions at the time of diagnosis. These indicators and the associated costs would be important for the clinical staff and clients to decide the appropriate treatment plan. To the best of our knowledge, no similar study has been conveyed prior to the publication of this paper.
MATERIAL AND METHODS
This longitudinal study used retrospective data from cats diagnosed with HT and followed up between 2010 and 2019 from the Blythwood Veterinary Group in NE London. Feline patient records contained information including date of birth and diagnosis of HT. The diagnosis was based on the patient’s history, clinical findings, and laboratory findings of high T
4 concentrations. In HT-suspected cases where T
4 was below 66 nmol/L, another measurement (equilibrium dialysis) was performed 4 to 6 weeks later from a new sample (13.2% of the cats). The range of observations varied between 37 and 90 nmol/L for total T
4 concentration (reference intervals 30 to 60 nmol/L), and between 58 and 162 nmol/L for serum T
4 (reference intervals 12 to 50 nmol/L). Only cats that either began MM or underwent ST were included in the study. They were subject to one treatment only. Cats with renal and liver disease or cardiac murmur that were included in the study had been diagnosed for these conditions before the HT diagnosis. Response to treatment (MM or ST) was evaluated by a decrease in the measurement of T
4 and only cats that reacted positively to treatment were included (concentration of T
4 below 50 nmol/L in 4 weeks, 40 nmol/L in 12 weeks, or 30 nmol/L in 24 weeks after treatment initiation).
Survival time from the date of diagnosis was calculated in months. Most cats were euthanized when their quality of life reached an unacceptable level due to a variety of conditions (decision taken by agreement between clinical staff and owners). Death was recorded on the date when either the cats died from natural death or were euthanized. Cats that were not entirely monitored or remained alive at the end of the study were marked with ‘last seen alive date’ or ‘data collection date’, respectively.
The animals included in the study were subject to routine veterinary procedures and therefore, no ethical considerations were raised. The patient and owner’s confidentiality was provided in all cases.
The following categorical variables were used: breed (domestic short or and pure breed), the enzymatic activity of ALT (high or normal), chronic kidney disease (CKD) (present or absent), cardiac murmur (present or absent), gender (male or female), and treatment (MM or ST). Age at the time of diagnosis (month) was used as a covariate. Different pure breed cats (Maine Coon, Russian Blue, Siamese, Persian, Chinchilla, British Shorthair, Abyssinian, Burmese, and Himalayan) were small in number and were therefore included under the same category ‘pure breed’.
A multivariate Cox regression analysis was implemented. The final adjusted model was obtained via a backward stepwise selection of variables following the Wald test (significance P<0.05). The -2 Log Likelihood test was used to evaluate the fitness of the adjusted model. The interactions between the significant variables were added to the model and tested for significance. Plots of partial survival curves for the different levels of the categorical variables were produced. The analysis was performed using the survival analysis tool of the software IBM® SPSS® Statistics 21.
RESULTS
A total of 136 cats were included in this study. A high proportion of cats were euthanised (n=93, 68.4%), only five (3.7%) died naturally. Thirty-eight cats (27.9%) were not continuously followed by the end of the study. The following descriptive results were obtained: breed (domestic short hair n=110, 80.9%; pure breed n=26, 19.1%), enzymatic activity of ALT (high n=25, 18.4%; normal n=111, 81.6%), CKD (present n=23, 16.9%; not present n=113, 83.1%), cardiac murmur (present n=21, 15.4%; not present n=115, 84.6%), gender (male n=61, 44.9%; female n=75, 55.1%), and treatment (MM n=104, 76.5%; ST n=32, 23.5%).
A cox regression was successfully fitted to the data with a -2 log likelihood of 791 (P<0.001).
Table 1 summarizes the median survival statistics for the variables which were found to be significantly different. There was no significant difference in median survival time between the MM or ST therapies (calculated for both as 23.5 months with interquartile range (IQR) 6.9, 48.0, and P=0.403). Cardiac murmur and gender were also not significantly different (P>0.05).
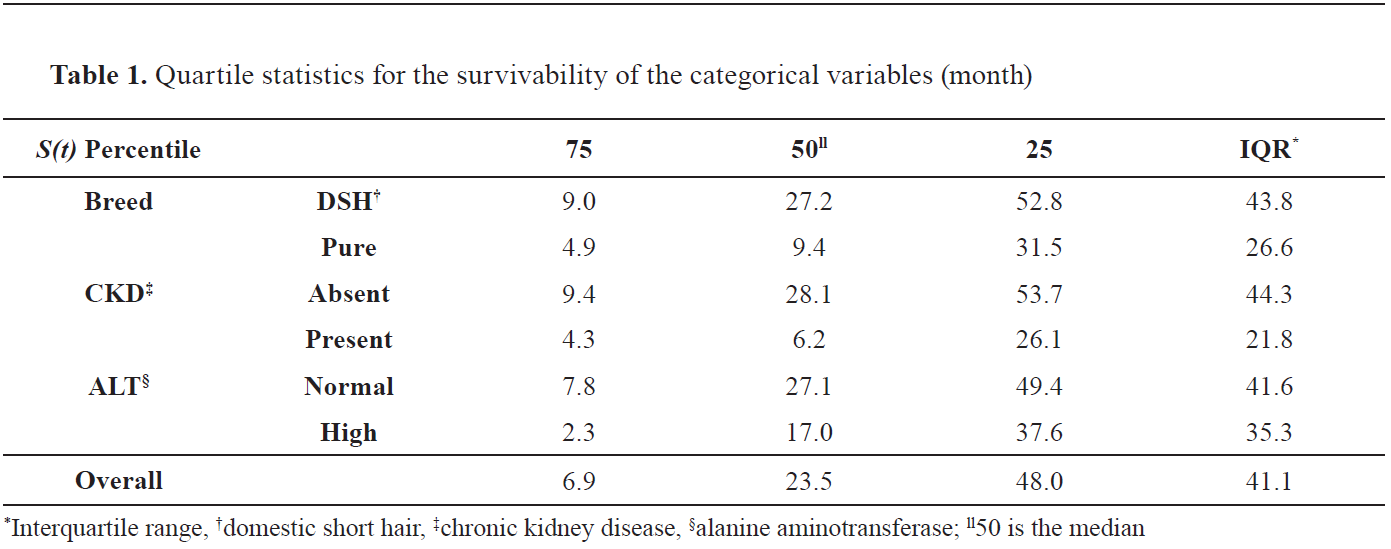
There was a significant difference (P<0.05) in survival between ‘domestic short hair’ (DSH) (median 27.2 months) and ‘pure breed’ (median 9.4 months); HT DSH cats had a lower hazard death rate and therefore increased survival rate. Cats with HT and CKD showed lower survival times compared to cats with HT but without CKD (median 6.2 vs. 28.1 months, P<0.001). The hazard death rate was higher in cats with both HT and CKD. Cats with both HT and high activities of ALT showed a lower survival time compared to cats with HT and normal activities of ALT (median 17.0 vs, 27.1 months, P<0.01). The death rate was higher in cats with HT and high ALT activities.
The interaction between the variables (high ALT and CKD) was correlated with a significantly lower hazard death rate, therefore suggesting the inexistence of a full additive effect of these conditions in the survivability of cats with HT.
The age at diagnosis was 15.2 years (median, IQR 8.0, 17.1) or 15.3 years (mean, 95% CI, 14.7, 15.9). Age at diagnosis had a significant (P<0.001) negative correlation with survival time (positive correlation with hazard death rate), increased mortality (1.008%/month, or 12.096%/year). The mean age at death was 17.6 years (mean, 95% CI, 16.9, 18.2).
Figures 1,
2, and
3 plot the partial survival functions of these same variables, indicating the survival and hazard death rates.
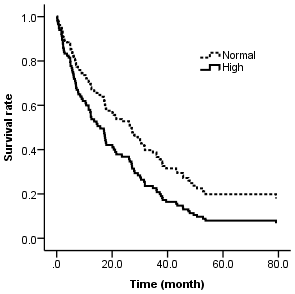 Figure 1.
Figure 1. Survival function for cats after hyperthyroidism treatment (pharmacological or surgery), with high or normal activities of alanine aminotransferase (ALT). Partial plot at median values for the other variables. The hazard (death) rate is the complementary of the survival rate. In the legend ‘Normal’ refers to normal activities of ALT and ‘High’ refers to high activities of ALT
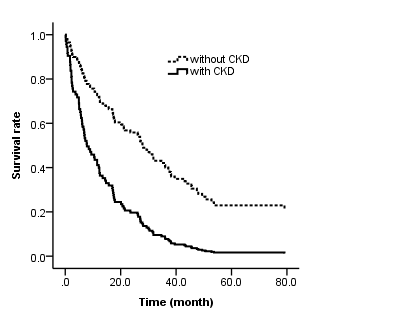 Figure 2.
Figure 2. Survival function for cats after hyperthyroidism treatment (pharmacological or surgery), with and without chronic kidney disease (CKD). Partial plot at median values for the other variables. The hazard (death) rate is the complementary of the survival rate
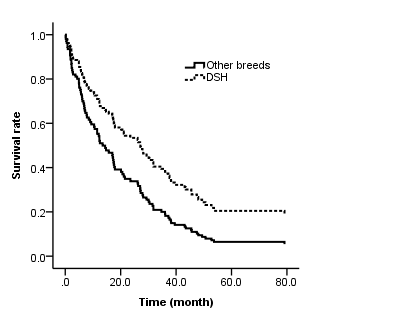 Figure 3.
Figure 3. Survival function for cats after hyperthyroidism treatment (pharmacological or surgery), of Domestic Short Hair (DSH) and pure breeds. Partial plot at median values for the other variables. The hazard (death) rate is the complementary of the survival rate
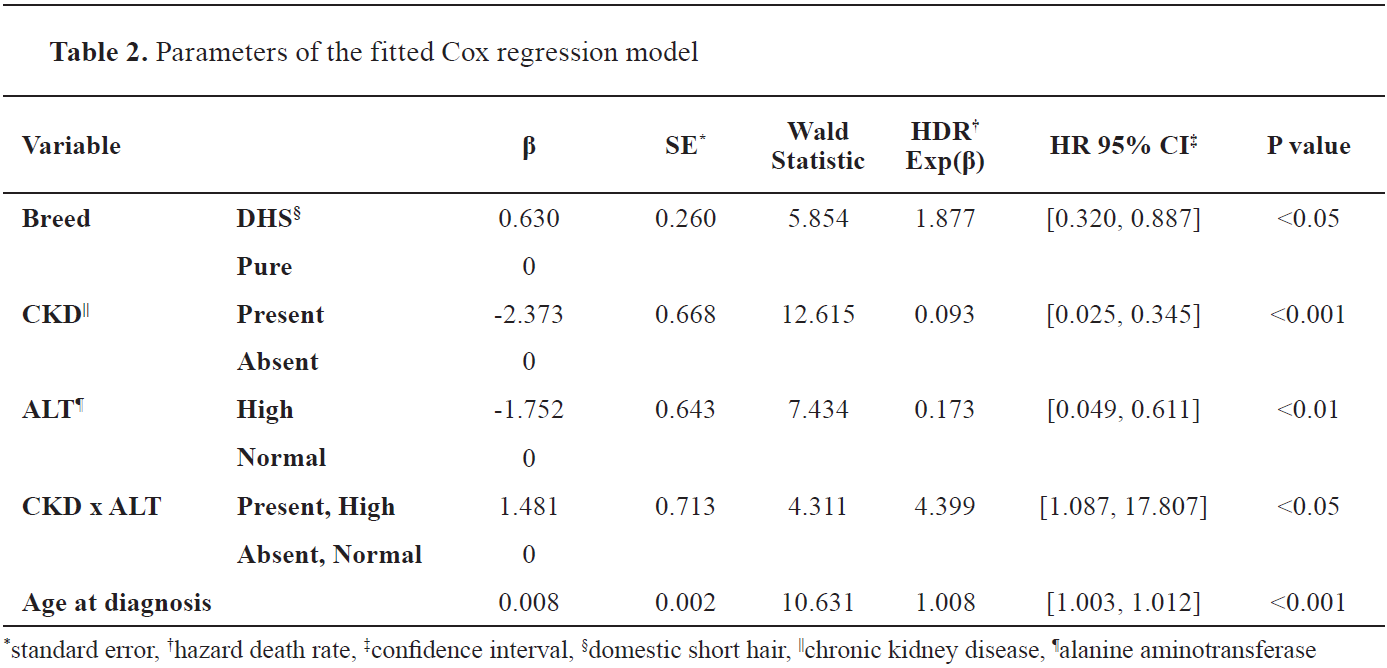
The full description of the coefficients in the model is summarized in
Table 2. Given the parameters in
Table 2, the hazard function, h(t), was calculated as:
h(t) = exp (0.630X
1 – 2.373X
2 – 1.752X
3 + 1.481X
4 + 0.008 ×
age at diagnosis)
where:
h(t) is the life expectancy (month);
X
1, X
2, X
3, and X
4 are the dummy variables taking the value ‘1’ respectively for DSH breed, presence of CKD, high ALT, and interaction between the presence of CKD x high ALT (in the other situations taking the value ‘0’; thus, eliminating the parameter from the equation).
DISCUSSION
The results of this study showed no difference in survival between surgical or methimazole treatment for HT, therefore when owners and veterinarians are deciding on treatment, the decision should be based on other factors.
The median survival time for cats receiving MM or ST (23.5 months) is similar to the median survival found by other authors, 24 months (
9) and 25 months (
10) in cats treated by
131IT, and 24 months for cats treated with MM (11). This contrasts with 13.8 months (13) and 36 months (12) reported by others.
The age at diagnosis (median 15.2 years) in the current study was similarly reported at 14.3 years in another paper (
13). The age at diagnosis and the average age at death were reported for 2.3 and 2.6 years higher than in the current study, respectively (
10). This can be explained by the generally higher life expectancy in the last two decades of life due to the better health care provided to the cats (
1, 15).
The survival was negatively correlated with the age at diagnosis, as could be expected with the increasing HR at the rate of 1.008%/month.
Our study found no female gender predisposition for the development of HT in cats which was in agreement with another report (
3). Other studies (
10, 16) reported that females have a higher predisposition. Further studies are required, including details of neuter status.
This study offers novel information about the breed difference in survival for cats with HT, indicating that the domestic shorthair cat has a survival advantage compared to the pure breeds (DSH median 27.2 months, pure breed median 9.4 months). Pure breeds of cats have lower genetic diversity due to the higher inbreeding coefficients when compared to randomly bred cats (
17). Benson et al. (
18) reported that higher inbreeding coefficients were associated with decreased individual fitness and disease resistance in wild felid populations. It is therefore hypothesized that the results of our study may be attributable to the heterozygosis effect, as exotic breeds have a higher inbreeding coefficient which may decrease the survival.
Until recently it was thought that breed was not a contributory factor to an increased HT risk, however, three different epidemiological studies (
16, 19, 20) showed that exotic breeds of cats (Himalayan, Siamese, Persian, Burmese, Birman, Chinchilla, Cornish Rex, and Tonkinese) may be at decreased risk of developing HT. Although this appears to contradict our results, the development of HT and survival beyond HT treatment may involve different traits. Our sample size per breed was too small for individual breed analysis, hence these findings and hypotheses require further investigation.
It could be expected that the cardiac disease would affect the survivability of cats diagnosed with HT. HT is associated with secondary hypertension and can be comorbid with cardiac disease (
8, 21), with the existence of an eventual difficulty in the control of hypertension due to the association between HT and systemic vascular resistance (
13). This was not found to be a significant variable affecting survival in the present study. Further details on cardiac screening and the presence of hypertension pre-HT treatment would be useful for in-depth analysis of this finding, but data were not available.
CKD is a common disease in geriatric cats (
21) increasing with age and affecting 30% of cats over 15 years old (
22), or 31% according to Boyd et al. (
23). As HT is also prevalent in geriatric cats, clinical signs are likely to overlap (
24) and CKD may be comorbid with HT (
25). It has been suggested that pre-existing CKD affects the survival of cats treated for hyperthyroidism (
11). Our study supports the hypothesis that cats’ survival with HT and CKD has a median of 6.2 months, whereas cats with HT and no CKD survived a median of 28.1 months, which tallies with the median of 5.9 months with and 20.1 without CKD (13). The current study was also in agreement with the findings of Milner et al. (
11) studying
131I treated cats. The interrelationship between renal and thyroid function has been discussed in humans. In a report of den Hollander et al. (
26) it was concluded that the kidney is an important target of thyroid hormone action and that renal function decreased during treatment of HT. Future studies in cats should explore the duration of CKD pre-HT diagnosis as a variable of survival.
Boyd et al. (
24) reported that CKD is diagnosed on average at 12.8 years 95% CI (12.2, 13.4) and has a typical survival time of 25.5 months. This is slightly below the average age of diagnosis (15.3 years) and the survival time (28.1 months) reported in our study for HT cats. The 12-year difference (2020–2008) between the reports of Boyd et al. (
23) and our study may be explained due to the increased life expectancy and better health care for geriatric cats (
1, 15).
It has been reported that cats treated for HT tend for higher activities of ALT (
15). The current study corroborates this finding, which can be explained by the action of MM, promoting increased ALT activities (
27). The synthesis block of the thyroid hormone can also be disturbed by liver pathologies (
8), but our data showed that when high activities of ALT and HT are comorbid, they did not increase the hazard death rate in the same proportions. However, many cats have increased activities of ALT unrelated to MM.
It could be hypothesized that the presence of comorbid conditions could significantly reduce the survival time of cats with HT, or accelerate both conditions, thus enhancing hazard death rates. The present study showed that the increase of the hazard death rate (i.e. decrease in survival by using a Cox regression model) was not cumulative.
Survival times are important estimations in order to give
a priori information to the veterinarian and cat owner, to allow an informed decision of treatment choices, and to manage the owner’s expectations. The treatment costs may vary between £700 (excluding Value Added Tax, VAT) for one-off surgery (excluding additional complications but including medication, hospitalization, and blood tests) and £665 (excluding VAT) per annum for the medication (including recheck appointments and blood tests) (
28).
The results of our study provide new information regarding the cats’ survival treated for HT given a range of other variables. However, while it was a convenient sampling design that captured all the available information with relatively good sample size, it had some limitations, and these are outlined below. This study was restricted to retrospective data which prevented cases from being standardized and randomized by group, although the data came from a group of veterinary clinics, so the statistical effect of ‘local’ sampling is mitigated. While it is acknowledged that several diseases can be present but undiagnosed in geriatric cats with confounding results, significant associations were found. The date of death in euthanized cats was based on owners’ and veterinarians’ perceptions of life quality and may have been influenced by economic constraints. This lack of standardization is also a possible confounding factor (
23). The cause of death and the reason for euthanasia should be recorded in future studies (e.g., liver disease, recurrent hyperthyroidism). In our study, the number of animals sampled with and without conditions comorbid to HT was unbalanced, as was the number of cats treated by MM and ST. More balanced designs would be beneficial to avoid potential bias.
Despite the identified limitations, this preliminary study has produced significant findings worthy of more in-depth investigation, which may prove useful to consider in the management of cats with HT.
CONCLUSION
The non-significant difference of survival between surgical and methimazole treatment in HT-diagnosed cats means that the veterinarian/ owner’s decision for treatment would not render significantly different survival rates. A surgery may be preferred in cases when life expectancy is higher, thus avoiding complications related to treatment compliance and possible side effects. Considering the costs involved, the surgery, may also be the treatment of choice from a financial perspective if the life expectancy is higher than one year.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
We would like to thank the Blythwood Veterinary Group for the collaboration and collection of data. The research was conducted as a part of the project (Ref. No. UIDP/05937/2020).
AUTHORS’ CONTRIBUTIONS
FM was leader of the project. FM and RB contributed in all aspects of research and writing.

 10.2478/macvetrev-2022-0015
10.2478/macvetrev-2022-0015




