The combination of immunomodulators and antibiotics in the treatment of animals with diseases of bacterial etiology is one of the effective strategies for animal therapy. The drug gentabiferon-B combines antibiotic gentamicin and speciesspecific (bovine) recombinant interferons -α and -γ. The study aimed to evaluate the effect of course application of gentabiferon-B on the cytogenetic stability of bone marrow cells of outbred mice after administering mitomycin C (MMC). The proportion of polychromatophilic erythrocytes in the bone marrow was assessed. There was no effect of gentabiferon-B on the frequency of polychromatophilic erythrocytes with micronuclei in both healthy animals and mice with MMC-induced cytogenetic instability. The course application of gentabiferon-B before the administration of MMC led to an increase in the proportion of polychromatophilic erythrocytes (46.03±2.61%) which was non-significantly different than the negative control group. The administration of MMC alone caused a decrease in the proportion of polychromatophilic erythrocytes to 33.33±1.83%. The antitoxic effect of gentabiferon-B led to an increase in the level of polychromatophilic erythrocytes by 38.1% compared to the group that received only MMC. Studies have shown that gentabiferon-B does not have mutagenic activity and anticlastogenic properties, however, it reduces the toxic effect of MMC. In conclusion, it is indicative that gentabiferon-B has antitoxic properties and can be safely used in animal therapy.
Owing to agricultural intensification, the animals are constantly exposed to many stress factors of both physical and biological nature leading to an ever-increasing load on a living organism. The constant contact with various pathogens increases this load and under certain conditions it leads to the emergence of numerous infectious diseases in animals. At the same time, the use of antimicrobial drugs in veterinary practice is often on a large scale, and in many cases uncontrolled, entailing the formation of antibiotic-resistant forms of microorganisms. This leads to a gradual decline in the effectiveness of antimicrobial agents and a global spread of antibiotic resistance (
1).
Additionally, secondary immunodeficiency in productive animals could occur under numerous unfavorable factors including the irrational use of drugs. The treatment may include antimicrobial therapy and stimulation of the natural resistance of the animal’s body. Therefore, to increase the effectiveness of chemotherapy, it is advisable to use complex drugs with antimicrobial and immunomodulatory activity (
2).
One of these combined drugs is gentabiferon-B that contains the antibiotic gentamicin and speciesspecific (bovine) recombinant interferons -α and -γ (IFN-α, -γ) (
3).
According to the existing legislative norms, all new drug combinations must undergo a preclinical study to assess for potential toxic properties and to determine the spectrum of possible side effects and the conditions for their occurrence (
4, 5). Thus, despite the positive effects of antibiotics such as aminoglycosides, this group of antimicrobial agents has numerous side effects, in particular, a pronounced nephrotoxic effect, which is associated with the formation of reactive oxygen species (ROS) (
6, 7, 8). Moreover, ROS are known inducers of mutations in animal cells (
9).
In addition, the researchers discovered a wide range of biological activity of IFNs that also requires study when they are used as part of a complex drug. Moreover, the study of the physiological effects of the action of recombinant bovine IFNs is possible using the mouse as a model organism since the cross-species activity has been demonstrated in various studies (
10, 11). In earlier studies, the antimutagenic effect of IFNs has been demonstrated by their ability to induce apoptosis of cells with damaged DNA and to activate reparative processes (
9, 12, 13). The presented data suggest the possibility of the drug to affect the genotoxic ability of experimental mutagens such as mitomycin C (MMC) that may be of scientific interest. MMC, an aziridine-containing agent derived from
Streptomyces caespitosus or
S. lavendulae, is used as a chemotherapeutic agent for tumors of various etiologies, as well as an experimental mutagen (
14). The drug acts by two mechanisms: bioreductive alkylation of nucleic acids with the formation of DNA crosslinks, and by the generation of free radicals, such as superoxide and hydroxyl radicals, following metabolic activation (
14, 15). The main side effects of MMC are bone marrow suppression, pulmonary fibrosis, and kidney damage. In addition, MMC is capable of producing mutagenic and toxic effects which are expressed by inducing micronuclei and a decrease in the proportion of polychromatophilic erythrocytes in the bone marrow (
14).
The effect of gentabiferon-B on the MMC toxicity and the comparison of its properties with gentamicin and biferon-B which contains recombinant bovine interferons -α and –γ, may be of particular scientific interest. We hypothesized that gentabiferon-B will reduce the toxic and mutagenic effects induced by gentamicin and MMC in mice.
In this regard, the study aimed to determine the effect of gentabiferon-B on the cytogenetic stability of mouse bone marrow cells and to assess its anticlastogenic and antitoxic properties induced by MMC.
MATERIAL AND METHODS
Experimental animalsWhite outbred mice (n=60) weighing 20.0±2.0 g from the vivarium of FSBSI “All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy” (FSBSI “ARVRIPP&T”) were used as a biological test system. The experimental animals were kept in standard vivarium conditions (air temperature +18-23 °C, relative humidity 45-60%). Animals were given free access to water and feed.
All procedures with animals were preliminarily reviewed and approved at a meeting of the Bioethical Commission of FSBSI “ARVRIPP&T” before the onset of experimental work (Protocol No. 5-05/21 of 31.05.2021) and complied with the rules adopted by the European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (ETS 123), Strasbourg, 1986; Directive 2010/63/EU of the European Parliament and of the Council of the European Union of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; Guide for the Care and Use of Laboratory Animals. Washington (DC) 1996; Code of Ethics of the Veterinarian of the Russian Federation, recommended at the XIII Moscow International Veterinary Congress of the Association of Practicing Veterinarians of Russia, 2005.
Experimental drugsThe study drug was gentabiferon-B (LLC Scientific and Production Center ProBioTech, Belarus), which contains an antibiotic of the aminoglycoside group - gentamicin, in the form of gentamicin sulfate, 0.04 g for the active ingredient, and species-specific (bovine) recombinant interferons -α and -γ (IFN-α, -γ), total antiviral activity of at least 1x104 IU/ml (3). Comparative drugs were biferon-B (Scientific and Production Center ProBioTech LLC, Belarus), containing at least 1x104 IU of the total antiviral activity of a mixture of proteins of bovine recombinant interferons –α and –γ in 1 ml of a biological product, as well as a solution of gentamicin sulfate 4% (LLC SPE “AGROFARM”, Russia). The drug Mitomycin-C Kyowa (Kyowa Hakko Kogyo Co., Japan) containing mitomycin as an active ingredient was used as a positive control drug.
Experimental designThe following groups of experimental animals were formed. Group I (negative control) - the animals were injected intramuscularly (i.m.) with a single dose of sterile isotonic sodium chloride solution in a volume of 0.2 ml. Group II – the mice were injected i.m. with gentabiferon-B in a single dose corresponding to 1/10 LD50 (549.63 mg/kg) in a volume of 0.2 ml. The animals of group III were injected i.m. with Biferon-B in a single dose of 500 mg/kg (1/10 of LD50) in a volume of 0.2 ml. The animals of group IV were i.m. injected with gentamicin in a single dose of 486 mg/kg (1/10 LD50) in a volume of 0.2 ml. The mice of group V were four times injected i.m. with gentamicin at a therapeutic dose of 3 mg/kg in a volume of 0.2 ml, with an interval of 24 h. The animals of group VI were four times injected i.m. with gentabiferon-B at a therapeutic dose of 0.1 ml/kg in a volume of 0.2 ml, with an interval of 24 h. The mice of group VII were given injections of gentabiferon-B similarly to group VI, however, after the fourth injection of gentabiferon, the animals were once injected intraperitoneally (i.p.) with MMC at a dose of 2 mg/kg. The animals of group VIII were injected i.m. with biferon-B in a therapeutic dose (0.1 mg/kg) for four days, with an interval of 24 hours, and then they were injected i.p. with MMC at a dose of 2.0 mg/kg. In group IX, the mice were injected i.m. with gentamicin 4% for four days, with an interval of 24 hours (3.0 mg/kg), and then they were injected with MMC as in the other groups. The animals that received a single i.p. injection of MMC in a dose of 2 mg/kg (group X) were used as a positive control. Six animals were examined in each group. Removal of mice from the experiment was carried out 24 hours after the last injection by an overdose of carbon dioxide.
Micronucleus testThe frequency of micronuclei (micronucleus test) of polychromatophilic erythrocytes (PCE) in the bone marrow of mice is one of the most commonly used methods with high effectiveness (
16). The obtained bone marrow cells were added to 1% albumin solution in Hanks buffer saline solution (pH 7.4) (
17) and applied to glass slides. The samples were dried, fixed with methanol, and stained according to Romanowsky-Giemsa (
16). The study of bone marrow samples was performed at ×1000 magnification. The frequency of micronuclei was assessed per 1000 PCE; a total of 2000 PCE was evaluated per animal. We also took into account the proportion of PCE per 500 PCE and normochromic erythrocytes (NE), which can be used as a marker of toxicity of the studied drugs (
4).
Statistical processingStatistical data processing was performed using STATISTICA 10 and STADIA 8.0 software packages. The comparison of the samples was carried out using the van der Waerden χ-test at a significance level of p≤0.05 since the studied parameters did not correspond to the normal distribution. The results obtained were presented as the arithmetic mean (M) ± standard error (SE).
RESULTS
Study of mutagenic propertiesThe level of micronuclei in PCE of the bone marrow of mice was assessed in the studied groups (
Fig. 1).
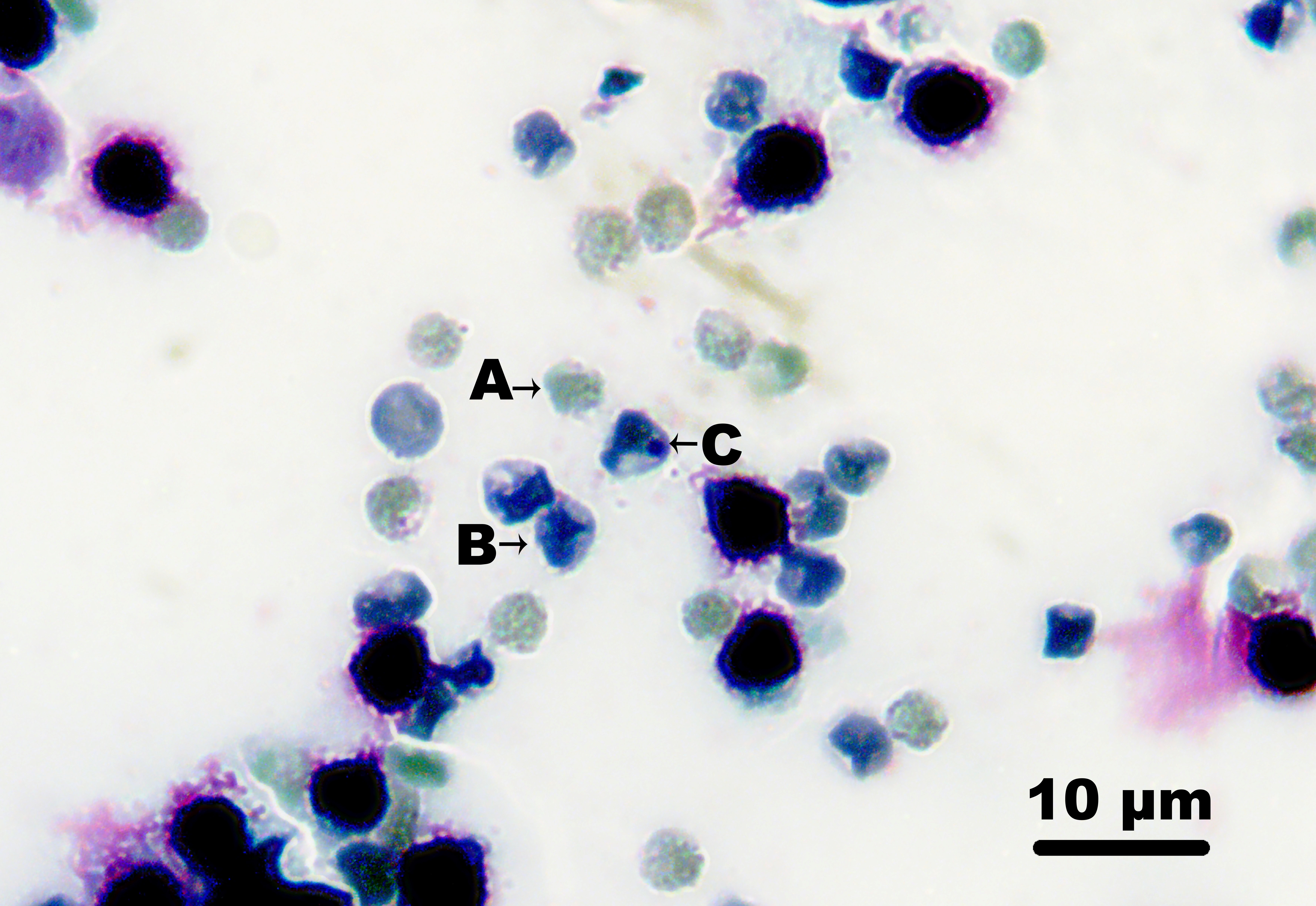 Figure 1.
Figure 1. Micrographs of the sample of mouse bone marrow: A-normochromic erythrocyte; B-polychromatophilic erythrocyte; C-polychromatophilic erythrocyte with a micronucleus. Magnification × 1000 (Bioskop-1 microscope, Russia)
According to the obtained data, the administration of the drugs gentamicin, biferon-B, and gentabiferon-B did not induce a statistically significant change in the frequency of PCE with micronuclei compared to the negative control group (group I), which was 0.40±0.08%. Thus, the frequency of PCE with micronuclei at a single injection of high doses of drugs corresponding to 1/10 LD50 was equal to 0.48±0.08, 0.34±0.07, and 0.54±0.19% in groups II, III, and IV, respectively (
Fig. 2).
 Figure 2.
Figure 2. Frequency of polychromatophilic erythrocytes with micronuclei in the bone marrow of mice: MNPCEfrequency of cells with micronuclei per 1000 PCE, %; I–X-numbers of experimental groups; a-statistically significant difference from the negative control group (p≤0.05); M±SE%-arithmetic mean ± standard error %
The course administration of gentamicin and gentabiferon-B did not cause any changes in the frequency of PCE with micronuclei. Thus, the frequency in groups V and VI was 0.20±0.07% and 0.34±0.07%, respectively. An increase in the frequency of PCE with micronuclei compared to the negative control group was observed with the introduction of an experimental mutagen MMC. Thus, in groups VII, VIII, IX, and X, the frequency of PCE with micronuclei was 2.03±0.34, 2.50±0.27, 2.60±0.17 and 2.80±0.22%, respectively. The course administration of gentabiferon-B before the intraperitoneal injection of MMC did not cause a statistically significant decrease in the frequency of micronuclei in the bone marrow cells of mice, same as with the administration of biferon-B and gentamicin.
Study of toxic effectThe proportion of PCE with the erythrocytes of other types in the bone marrow of mice was assessed in the studied groups (
Fig. 3). The proportion of group I PCE (negative control) was 49.83±2.15%. High and course doses of gentabiferon-B and biferon-B did not affect the PCE content in the bone marrow of mice. Thus, in groups II, III, and VI, the level of PCE did not differ significantly compared to the negative control group (50.45±2.26, 49.98±3.14, 44.49±3.86%, respectively). At the same time, the administration of gentamicin at a high dose (Group IV) induced a decrease in the proportion of PCE to 36.12±3.69% compared to group I. The PCE level with the course administration of gentamicin (Group V) was 34.27±6.60% and was significantly different compared to the negative control group.
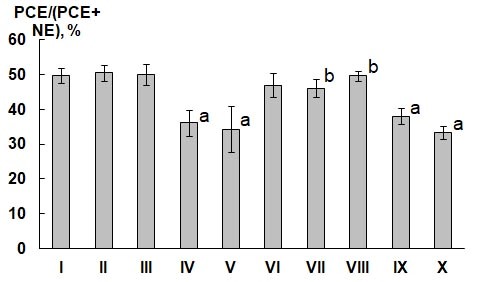 Figure 3.
Figure 3. The proportion of polychromatophilic erythrocytes relative to normochromic erythrocytes in the bone marrow of mice: PCE/(PCE + NE)-the frequency of polychromatophilic erythrocytes per 500 normochromic and polychromatophilic erythrocytes, %; I-X-numbers of experimental groups; a-statistically significant difference from the negative control group (p≤0.05); b-statistically significant difference from the positive control group (p≤0.05); M±SE%-arithmetic mean ± standard error
The administration of MMC in group X (positive control) led to a significant decrease in the proportion of PCE in the bone marrow of mice, which was 33.33±1.83%. In addition, injections of MMC and gentamicin (group IX) also caused a decrease in the proportion of PCE to 38.00±2.32%. By using gentabiferon-B and biferon-B together with MMC (groups VII and VIII), the PCE level was 46.03±2.61 and 49.58±1.50%, respectively, which was significantly higher than the values of the positive control (group X), and similar to the values of group I (negative control).
Thus, a decrease in the proportion of PCE in the bone marrow of mice was observed when they were injected with gentamicin and MMC, while single or course injections of gentabiferon-B and biferon-B, including together with MMC, did not lead to a decrease in the level of PCE. This may indicate that gentabiferon-B had no toxic effect on proliferating bone marrow cells or a protective effect against cytotoxicity induced by MMC.
DISCUSSION
Gentamicin was previously tested in several
in vitro genotoxicity studies (Salmonella microsomal analysis, mitotic crossing over test, gene conversion, DNA repair, Rec analysis), and in most of them, gave negative results. However, the mutagenic effect of gentamicin was noted in
in vitro tests on
Escherichia coli at the cytotoxic dose level, in a test for chromosomal aberrations in mouse L-cells, and in a test for the sister chromatids exchanges in human fibroblasts (
18). Another study demonstrated an increase in the frequency of chromosomal aberrations in the bone marrow cells of mice treated with intraperitoneal injections of gentamicin at a dose of 80 mg/kg per day for 2 weeks (
19). In addition,
in vitro studies demonstrated the genotoxic effect of gentamicin on the LLC-PK1 cell culture, assessed by the DNA comet assay (
20). To some extent, these effects can be explained by the ability of gentamicin to induce free radical oxidation (
21). A report by Bustos P.S. et al. (2016) showed an increase in the level of ROS in rat blood cells even one day after the intraperitoneal administration of gentamicin at therapeutic doses, intensification of lipid peroxidation processes, and a change in the activity of superoxide dismutase and catalase (
22). Although gentamicin has shown positive results for mutagenicity in some tests, these results have not been confirmed by a series of well-conducted genotoxicity trials: two
in vitro tests (analysis of chromosomal aberrations in CHO-K1 cells, analysis of CHO/HGPRT gene mutation) and one
in vivo test (micronucleus test on mice). Therefore, the European Medicines Evaluation Agency concluded that the genotoxicity of gentamicin is unlikely (
18). Several
in vitro and
in vivo studies also do not show the mutagenic effect of various types of recombinant interferons, including IFN-α and IFN-γ (
23, 24). Our data confirm the absence of mutagenic properties in gentamicin, biferon-B, and gentabiferon-B, both when administered as a separate substance, and when used in a combined drug containing recombinant interferons. The nonsignificant change compared to the positive control in the level of micronuclei in bone marrow cells when using both gentabiferon-B and biferon-B where cytogenetic instability was induced by MMC indicates that there are no antimutagenic properties of gentabiferon-B. The presented data may be explained by the ability of interferons to exhibit antimutagenic activity, such as DNA repair, depending on the type of interferon and the genotype of the cell (
13, 25).
Several studies have demonstrated the toxic effect of gentamicin in animals. Thus, when rats were administered different doses of gentamicin for 30 days, a decrease in the concentration of erythrocytes, hemoglobin, and hematocrit was found in the group that received 63 mg/kg of body weight/day. Renal tubules necrosis, vacuolization of hepatocytes, and bone marrow hypoplasia were observed when a dose of 156 mg/kg of body weight per day was administered. At the same time, all changes tended to reverse development after a 30- day recovery period (26). Gentamicin is capable of blocking numerous ion channels in the cells and induces ROS in which iron ions play a key role (
27). Same as other aminoglycosides, gentamicin exhibits nephrotoxicity and ototoxicity (
27). The alkylating drug MMC can also have a toxic effect on proliferating cells (
14). The presented data indicate the toxic effect of gentamicin and MMC, which may be manifested by inhibition of the proliferative activity of the bone marrow cells and, consequently, a decrease in the proportion of PCE and NE. This was evident by the decreased PCE content by 27.5, 31.2, 23.7, and 33.1% in groups IV, V, IX, and X, respectively, compared to the negative control group (group I). At the same time, the use of gentabiferon-B and gentamicin in a single high dose (group IV), or in multiple therapeutic doses, did not lead to a statistically significant change in the PCE level compared to the animals of group I. It was noted that the course administration of gentabiferon-B (group VII) and biferon-B (group VIII) before the injection of MMC did not lead to a decrease in the frequency of PCE in the bone marrow of animals, while the level of PCE was significantly higher (by 38.10 and 48.75%) compared to the positive control group. The decrease in the toxic effect when using gentabiferon-B and biferon-B when using gentamicin and MMC can be explained by the influence of recombinant interferons (
28).
The interferons’ effect on hematopoietic cells in the bone marrow has not been fully elucidated because in some studies they have been reported to have cytotoxic or cell proliferative properties (
29). In one study, IFN-γ and IFN-α were reported to suppress hematopoiesis (
30). Other studies have reported that IFN-γ stimulates hematopoiesis by an increased sensitivity of cells to IL-3 and/or granulocyte-monocyte colony-stimulating factor (GM-CSF) (
29).
In vitro studies noted that IFN-γ inhibited the proliferation of CD34+CD38- cells in the primary culture of hematopoietic bone marrow stem cells, but did not reduce their survival rate (
29). Indeed, one of the effects of the action of interferons on various cells is the arrest of the cell cycle and a decrease in their proliferative activity (
31). The presented data may be evidence in favor of the antitoxic effect of interferons on cells that is exhibited by a decrease in the sensitivity of low-proliferating cells and by inducing reparative processes following MMC administration. MMC had a predominantly cytotoxic effect on actively proliferating cells and caused a cytostatic effect in cells with a low mitotic index (
32). At the same time, the sensitivity of cells to DNA damage changes during the cell cycle, and its arrest in certain phases, for example G2, can play a decisive role in the survival of cells after sublethal DNA damage by alkylating drugs (
33, 34). However, Wang X.Y. et al. (
35) in an
in vitro study of the effect of IFN-γ and IFN-α on MMC-induced cytotoxicity of human fibroblasts demonstrated an increase in cell death when using IFN together with MMC due to priming of the apoptotic pathway of cell death (
35). The differences with the current study may be explained by the use of fibroblast cell culture rather than bone marrow cells which may limit the effect of physiologically active substances formed in the cells, such as GM-CSF or IL-3, as well as other forms of IFNs. Thus, despite the hypothesized decrease in toxicity and the hematopoietic effect on bone marrow cells under the influence of MMC or gentamicin, the antitoxic mechanisms of IFN when used with gentabiferon-B or biferon-B remains unclear and require further research.
CONCLUSION
As a result of our studies, the following was consluded. The drug gentabiferon-B does not affect the cytogenetic stability of bone marrow cells according to the level of micronuclei in polychromatophilic erythrocytes, concluding that it does not have mutagenic properties. The course administration of gentabiferon-B together and MMC did not lead to a significant decrease in the frequency of PCE with micronuclei compared to the positive control, concluding that it did not have an anticlastogenic effect. The use of gentabiferon-B reduced the toxic effect of MMC on bone marrow cells, which may be evidence of the antitoxic properties of gentabiferon-B.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The authors thank the staff of the vivarium of FSBSI “ARVRIPP&T” represented by the Head of the vivarium Topolnitskaya A.V. for the help in organizing the study. The experimental work was carried out in accordance with the government assignment No. 122011200146-8 (FZMR-2019-0013).
AUTHORS’ CONTRIBUTIONS
SS, VG, GV, DS, NK and AK contributed to the study conception, design, material preparation, investigations, data collection and analysis, participated in writing the manuscript and approved the final version.

 10.2478/macvetrev-2022-0016
10.2478/macvetrev-2022-0016


