Artificial insemination (AI) as a part of assisted reproductive technologies represents the oldest and most widespread method used to accelerate genetic progress in all domestic animals. After its first implementation in ovine reproduction and almost 80 years afterward, AI is continuously used for improving the genetic merit, utilizing either fresh or shorttime chilled semen. Nevertheless, regardless of the semen used for insemination, the conception rate (CR) is still lower in comparison to natural service. At least two factors are commonly thought to limit the success of the AI and reduce the CR: (1) failure of placing the semen directly into the uterus due to the specific anatomic structure of the ewe’s cervix; (2) lower viability of ram spermatozoa during cryopreservation (<30% progressively motile spermatozoa after thawing). This review elaborates on recent studies that aimed to achieve acceptable CR through the implementation of cervical or intrauterine insemination: deep intracervical, intrauterine trans-cervical, and intracornual. Several hormonal treatments (oxytocin, estrogen, or prostaglandin) were evaluated on inducing cervical dilation that facilitates insemination. A comprehensive analysis was given to the effects of several antioxidants (GSSG, GSH, and cysteine) supplemented in ram semen-freezing media. Sex-sorted ram semen fertility rate results were presented from our studies.
Small ruminant farming represents an important economical component of the agricultural production in most countries of the Balkan region. It provides the domestic and foreign markets with dairy and meat commodities. North Macedonia has gained significant economic benefits by exporting lambs and dairy sheep products in the EU markets and other neighboring countries. Despite these agricultural advantages of small ruminant farming, there is a continuous negative trend in the sheep population in the Balkan countries, especially evident in the recent years. As a result, sheepherders are undertaking various approaches aiming to improve overall herd productivity. The main strategy is based on improving the animal husbandry, animal welfare, production, and reproductive efficiency (
1). Henceforth, the dominant traditional nomadic shepherding has been replaced with modern housing barns equipped with automated milking units or parlours, and computerized feeders. Several dairy sheep breeds (East Friesian, Awassi, Assaf, Lacaune, and Chios) with an annual milk production of more than 300 liters per lactation (lasting approximately 200 days) have been imported with anticipation to increase the production per animal. Finally, different reproductive programs employing assisted reproductive techniques have been implemented aiming to improve the reproductive efficiency of the herds. Apart of the aforementioned strategies, there is evident progress in raising dual-purpose sheep breeds (Merino Landschaft, Ile de France, and Suffolk), that have a higher weight gain and feed conversion compared to the domestic breeds.
The local, indigenous breed Pramenka, which exists in several subtypes (strains) throughout the Balkan region (from Slovenia to North Macedonia), produces an average between 60 and 80 liters of milk per lactation. Despite the low productivity, they are well adjusted to the environment and are genetically more resilient to diseases thus having greater health status compared to the other imported or cross-mixed sheep breeds. Therefore, it would be highly beneficial for local farmers to exploit the possibilities of “pure blood” breeding and to continuously improve their productivity. The existing reproductive-technology scientific approaches could be utilized for achieving these goals.
The following text gives a comprehensive analysis to the existing and most recent assisted reproduction methodologies in veterinary medicine. The aim of this article is to give a more clarified review of the most important methods that are applicable for
ex-situ conservation of biological diversity, and that have been demonstrated to improve the sheep reproductive efficiency on commercial farms.
ARTIFICIAL INSEMINATION IN SHEEP (AI)
Sheep are among the first domesticated animal species in which AI methods have been employed. However, currently AI is more frequently utilized in other animal species. Several European countries (including some Balkan countries) are still utilizing AI in the ovine reproductive programs, similarly as in dairy cattle. France, Spain, and Italy are routinely using AI primarily to improve the genetic merit of their local breeds (Lacaune, Manech, and Sarda dairy sheep, respectively). This yielded higher milk production in Lacaune, starting from 60 to more than 300 liters per lactation (
2).
The limitations of successful implementation of AI in sheep are low profitability, low economic impact, and lack of skilled practitioners. In our personal view, the financial impacts should not be limiting factors in biodiversity preservation programs where AI is a valuable and indispensable tool.T he success of AI depends on objective (breed, age, season) and subjective factors (AI method, AI timing, estrus detection rate, estrus synchronization, number of inseminations, preservation of gametes, animal health status) (
3). Additionally, feed quality must be considered as a paramount factor that might affect general health and reproductive status in the animals (
4).
The following text will introduce novelties in AI in respect of the 1) semen analysis (prior or following conservation), 2) semen preservation media, 3) insemination techniques, and 4) cervix dilatation (hormonal and medicament treatments).
Semen collectionSemen collection in rams is mainly performed by artificial vagina (AV) or electroejaculation (EE) (
Fig. 1). Spermatozoa can be successfully obtained from the epididymis in rams, which is reported as a novel technique for semen collection in the scientific literature (
5, 6). The success of AV semen collection largely depends on the animal’s experience and accommodation to the method. Indigenous ram breeds tend to be less compliant with this method if they are not properly trained (
7). In our experience, the success in AV semen collection in rams between ages of 8 and 12 months was 40.0% following training. This success rate was higher (87.5%) with rams between the age of 12 and 18 months (
8). Therefore, AI centers should start introducing rams to the AI semen collection method since the earliest age to achieve higher reproductive efficiency.
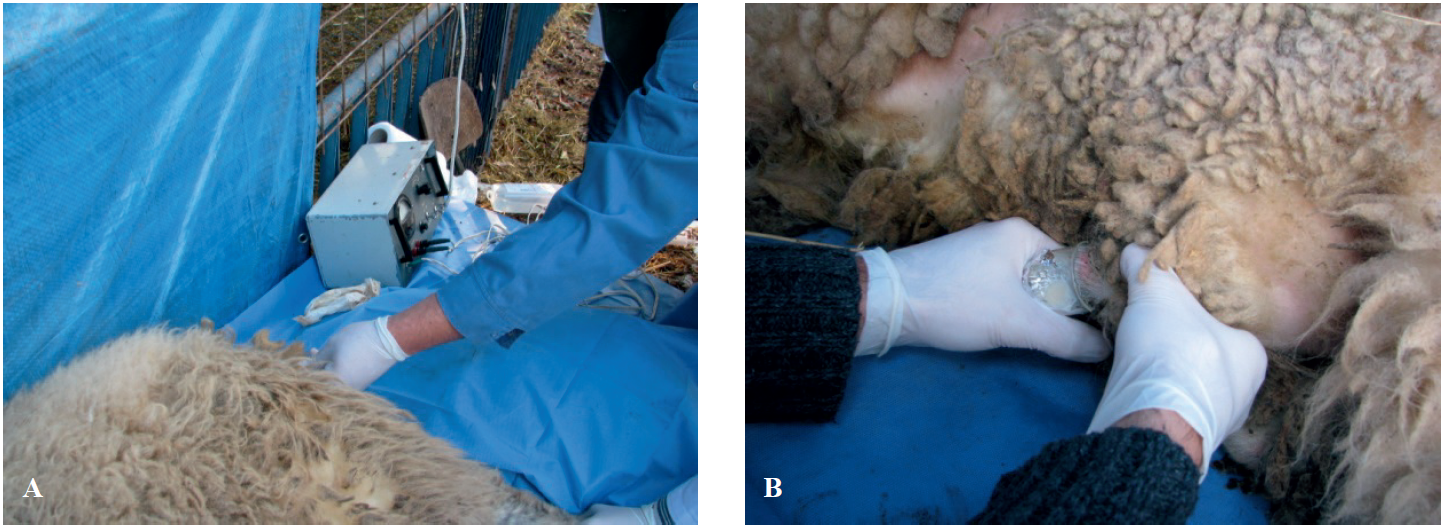 Figure 1.
Figure 1. Semen collection by electro-ejaculation (EE). Inserting the probe in the rectum of the ram (A); Collection of the ejaculate in sperm collector (B)
EE semen collection technique is usually applied when rams are not able to adapt to the AV or have decreased libido. Regardless of the used semen collection method, the criteria for the ejaculates are equivocal. Nikolovski et al. (
8) reported that the ejaculates obtained by AV have significantly higher motility (P<0.001), volume (P<0.01), and percentage of live sperm (P<0.01) compared to the ejaculates obtained by EE. Conversely, Ledesma et al. (
9) showed that EE ejaculates had a higher number of spermatozoa with intact membrane and functional mitochondria, increased percentage of seminal plasma, and total protein content, as well as abundance of low molecular weight proteins when compared to the AV ejaculates.
Similar to our finding, the Dutch center for Genetic Resources suggested that the semen extracted directly from the epididymis could be used for sheep AI, since it contains an average sperm count of 20x10
9/ml (
5, 6). Hence, an increased conception rate might be anticipated compared to semen collected either by AV or EE methods. The main disadvantage of this technique is that the semen could be taken only once, i.e., after castration or slaughtering of the animal.
In attempt to overcome the need for training and adjustment of young rams to AV method for semen collection, (
10) have designed a glass, roundbottomed (9.8 cm length, 1.9 cm inner diameter) vaginal collection vial device (VCV). The VCV was covered by a soft rubber cap with a hole in the center increasing the pressure exerted on the penis thus inducing ejaculation. The VCV was inserted into the ewe’s vagina and was bent at a 10° angle. Following the ejaculation, the VCV was withdrawn and the semen was processed for analysis. The results have shown that the likelihood of semen collection from untrained rams was significantly higher (P<0.01) compared to the AV method. In addition, the ejaculate volume, percentage of motile spermatozoa, or forward progressive motility score were not affected by the method and were not significantly different compared to the semen collected by AV. The authors concluded that VCV could be used to collect the semen from rams that are not trained for AV without affecting the semen quality.
Semen assessmentLaboratory assessment of semen may greatly differ from its actual
in vivo fertility. Moreover, the combination of various methods and laboratory settings may considerably affect the overall semen assessment score. Therefore, it is of utmost importance to achieve a consensual and complementary semen assessment assay which would yield highest predictive value. According to Graham (
11), bull semen fertility was correlated with sperm motility in the range of 0.15-0.84, with sperm morphology in the range of 0.06-0.86, and with sperm viability in the range of 0.33-0.66. Evaluation of the semen quality might be accomplished utilizing several
in vitro methods. The classical semen analysis includes sperm count, morphology, motility, plasma, and acrosome membrane integrity. Additionally, sperm penetration assay (
12), zona-binding or hemi-zona test (
13), and acrosome reaction assay (
14) are used as sperm functional tests which are employed to assess the spermatozoa ability to interact with the oocyte and can predict the
in vivo semen fertilization capacity to some extent (
15, 16). Sperm volume, consistency, density, color, and pH are the most common and traditional parameters used for general macroscopic assessment of the semen. They have moderate predictive power for the semen fertility but could indicate a major deficiency of the ejaculate. The traditional microscopic methods used are wave-like and individual sperm motility, sperm concentration, viability, and morphology.
Supravital staining can be performed with various stains and methods. In our experience, Hancock-2 stain has been confirmed to be effective for ram sperm (
17) but also for other animal species as well (
18). This is a one-step method which includes mixing aliquots of sperm and dye on a pre-warmed microscope slide, smearing, and drying. The method is based on the dye penetration through the damaged acrosome and/or plasma membrane. Sperm morphology can be assessed simultaneously. According to these assessment criteria, sperm cells can be categorized in five categories: category 1 – cells with intact acrosome, plasma membrane, and morphology, category 2 – cells with affected acrosome, but intact plasma membrane and morphology, category 3 – cells with affected plasma membrane, intact acrosome and morphology, category 4 – cells with affected acrosome and plasma membrane, and intact morphology, and category 5 – cells with affected acrosome, plasma membrane, and morphology (
17).
For kinetic and motility assessment of sperm, CASA (Computer-assisted sperm analysis) is used which may or may not include fluorescent-probe reading. The CASA system is composed of a phasecontrast microscope, camera, heated mini-plate, image digitizer, and data analysis software. The main purpose of the CASA is to analyze spermatozoa movements by restructuring the spermatozoon head trajectory expressed in values per unit of time. The yielded values are highly dependable on the CASA settings which can significantly impact the interpretation of the results. The software detects the spermatozoa by their contrast in relation to the background, pixel size, and pattern of motility, thus differentiating them from other non-cellular particles. If these settings are not unified between different laboratories for the same animal species and/or breed, the results may vary significantly. Additionally, the density and temperature of the medium, sperm concentration, as well as the chamber depth may contribute to variations in the sperm-analysis methodology. Therefore, each CASA report must be accompanied by this type of information so they could be reproduced in different laboratory settings. The spermatozoa trajectory is being recorded by several consecutive microphotographs (
19). With algorithmic estimations, the software yields various parameters which may not necessarily be used for individual interpretation. Most CASA system reports are including the following parameters: number of motile (tMOT) and progressively motile sperm (pMOT), curvilinear (VCL, μ/s), linear (VSL, μ/s), and average sperm trajectory velocity (VAP, μ/s), amplitude of lateral head displacement (ALH, μ), beat-cross frequency (BCF, Hz), trajectory straightness (STR, %) - estimated as VSL/VAP, and linearity (LIN, %) - estimated as VSL/VCL (
Fig. 2) (
17). In our latest research (
17) the ram spermatozoa kinetic parameters were acquired with the following CASA system (Hamilton Thorne v.12) settings: chamber depth - 20.7 nm; cell concentration - 50 x 106 in 1 ml of medium; medium specific weight - 1.039; medium temperature - 37 °C; frames-persecond – 60 Hz, frames – x30; minimum contrast threshold for cell detection – 60; minimum cell size threshold – 5 pixels; high velocity cells threshold – VAP ≥75 μ/s; static cells threshold – VAP ≤21.9 μ/s and VSL ≤6 μ/s.
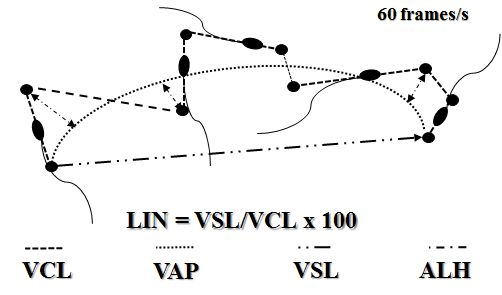 Figure 2.
Figure 2. CASA kinetic parameter estimation. VCL-curvilinear track velocity, VAP-average track velocity, VSL-straight-track velocity, ALH-amplitude of lateral head displacement, LIN-linearity index
The hypo-osmotic swelling assay (HOS) (
20) is routinely used for assessment of the functional integrity of sperm plasma membrane and serves as a useful indicator of the sperm fertility potential. The test is based on determining the ability of the sperm membrane to maintain equilibrium between the cell and its environment. The influx of the hypoosmotic fluid cause sperm swelling and tail coiling. The higher incidence of swollen sperm indicates intact and functional plasma membrane (
21).
Sephadex sperm filtration has been indicated as a plausible method for discarding unviable cells, cell debris, and other particles in the ejaculates which may affect its fertilizing capacity in rams (
22). There are several types of Sephadex (G-15, G-25, G50, G-100, and G-200) but G-15 has been demonstrated to have a high selecting ability for non-refrigerated and chilled ram semen (
22), whereas G75 for buffalo semen (
23).
Sperm proteomics is among the latest sperm assessment methods which employ molecular-level assessment in determining sperm motility. About 150 proteins were related to highly motile ram spermatozoa. PEBP4, SPATA18, and CPVL were determined as biomarkers for ram sperm with high motility. PEBP4 was located in the principal portion of the tail, whereas SPATA18 and CPVL were detected in the mitochondria. Another important protein located in highly motile spermatozoa was NUP98 which was located in the basket filaments in the nuclear pore complexes (X-28). It was suggested that the abundance of this protein in highly motile sperm cells was associated with a lower apoptotic rate. A higher abundance of CPVL, IZUMO1, and AGA was associated with increased sperm-egg fusion.
Flow cytometry (FC) is employed for the semen assessment that allows a large number of spermatozoa to be analyzed in short time intervals. FC detects both structural and physiological changes of the spermatozoa that usually cannot be detected by the classical methods. FC incorporates assays that evaluate the acrosome and spermatozoa membrane integrity, chromatin structure, membrane phospholipid structure, mitochondrial potential activity, cell membrane oxidation status, etc. For example, Sybr14 and propodium iodide is used for the dual staining technique of the chromatin, PNA-FITC and propodium iodide are used for assessment of spermatozoa plasma and acrosome membrane integrity, and acridine orange stain is used for the chromatin integrity (
24).
The acrosome reaction test assesses the functional ability of the spermatozoon to achieve structural modification of the acrosome cap by its fusion with the outer plasma membrane and releasing its constituents on the spermatozoon surface. There are several various methods reported in the literature such as naphtyl-yellow/eritrosin B, acridine orange-UV, Trypan blue-Bismark brown-Rose Bengal triple staining, chlortetracycline-UV, and
pisum sativum (pea lectin) (
25). Moce and Graham (
26), reported a method that first included inducing sperm capacitation by incubation in TALP and Krebs Ringer media at 37 °C and 5% CO2 followed by
in vitro acrosome reaction induced by Ca-ionophore A 23187, glycosaminoglycans, and follicular fluid. Following incubation in 2% trypan blue in PBS and fixation in 4% glutaraldehyde, the spermatozoa are stained with 0.8% Bickmark brown for 5 min and with 0.8% Rose Bengal in PBS for 30 min. The microscopic evaluation was determined by a phase-contrast microscope with an immersion objective including at least 200 sperm cells. Spermatozoa that underwent capacitation would have spilled acrosomal contents that would be stained with a light-rose tinge of the post-acrosomal and white acrosomal region. Live spermatozoa which did not undergo capacitation would have light-rose post-acrosomal and pink acrosomal areas. Unviable cells with defective acrosome would have blue post-acrosomal and white acrosomal areas (
27).
Sperm Chromatin Structure Assay - SCSA is a fluorescence cell sorter test, which measures the susceptibility of sperm DNA to denaturation after exposure to heat or low pH. Therefore, SCSA can assess the DNA status and sperm fertility (
28). Multiple studies have confirmed and suggested that sperm DNA damage was not associated with sperm motility while having a negative impact on fertility leading to impaired fertilization, slow early embryo development, reduced implantation, miscarriage, and birth defects in pluriparous animals (
29). Therefore, the SCSA has been validated as a highly useful test for determining male breeding soundness, especially in human reproduction.
The cervical mucus penetration test is used to assess the spermatozoa interaction with the cervical mucus. It incorporates the post-coital test, cervical mucus contact test, and cervical mucus penetration test. The post-coital test is conveyed 6-12 hours following intercourse. A drop of cervical mucus is sampled from the ewe and is assessed for consistency, pH, cellular composition, and ferning. If the cervical mucus has normal characteristics, then the number of motile spermatozoa is checked. The test is considered positive if at least 10 motile sperm cells are detected in each microscopic field. If there are no motile cells found in the abnormal composition of the cervical mucus, then the female factor is considered as the plausible cause for unsuccessful fertilization. In this way, both the male and female factors are considered simultaneously. This test is applicable in
in vitro conditions as well (
30). The cervical mucus contact test is performed by mixing a drop of cervical mucus and spermatozoa on a microscope slide, thus assessing the number of motile spermatozoa advancing in the mucus. If the cervical mucus contains sperm agglutinins, then the contact with the spermatozoa results in sperm-agglutination and unsuccessful penetration. Another variant of this test is the capillary tube test in which the cervical mucus is placed in a plastic tube (5 cm length, 3 mm width) and is vertically submerged in a semen sample.
Following incubation, the tube is placed under a microscope and assessed for the spermatozoa penetration distance in the tube. The distance of the most advanced spermatozoon in the tube following 90 minutes of incubation or the motile sperm count at 3 cm distance in the tube following 60 minutes of incubation can be used for the interpretation of this test.
Hamster zona-free ovum test is an expensive, laborious, complex, and highly variable in performance for different laboratory settings. Its advantage is the high sensitivity in assessing multiple spermatozoa functions such as capacitation, acrosome reaction, egg-fusion, and sperm chromatin decondensation. Hamster eggs are cleaved of cumulus cells by using hyaluronidase, trypsin, and merceptal. Sperm cells are capacitated in vitro by using glycosaminoglycans, Ca-ionophores, follicular fluid, yolk-buffer, platelet activation factor, and progesterone. The capacitated and acrosome-activated spermatozoa and zona-free hamster oocytes are placed in a fertilization medium and following incubation (3 hours, 38.5-39.0 °C, 5% CO2) the oocytes are washed, stained, and examined under the microscope (x400). If 10% of the oocytes are penetrated and each has at least 5 spermatozoa then this test is interpreted as positive (
31).
The hemi-zona test assesses the binding ability of capacitated sperm cells. It usually utilizes bisected oocytes, each half used for control-fertile and tested semen samples, respectively. The same hemi-zona surface for both specimens exclude the oocyte as a factor for successful fertilization with the assumption that they are structurally and functionally unaffected. In this way, if the control has positive and the test specimen has negative binding to the zona pellucida, then this test is interpreted as negative meaning that the capacitated sperm cells have not achieved a binding ability and are infertile (
32).
Semen conservation/preservationThe ram semen conservation can be performed by two classical methods: chilling (5-15 oC) or freezing (-120 oC). During the chilled-liquid semen storage, the spermatozoa metabolism is decreased and thus their lifespan is extended for 6-8 h. Nevertheless, exposure of the ram semen to lower temperatures significantly reduces the sperm viability due to its susceptibility to damage during cryopreservation. These deleterious effects can be alleviated by gradual decreasing of the temperatures (chilling) and by using semen extender/diluent containing cryoprotective components. Lecithin contained in the egg yolk or the soybean, has been widely used in various semen extenders as cryoprotective component (
33).
The chilled semen can be stored and transported at either 15 °C (most commonly in France) or 5 °C (most commonly in Australia, New Zealand, and South Africa). Detailed descriptions of both techniques may be found elsewhere (
34, 35). Thermo- or Styrofoam containers supplemented with frozen glacial acetic acid ampoules are usually used to store the chilled semen at 15 °C. Regardless of the type of the containers, a direct contact between the frozen ampoules and the semen is avoided by wrapping the ampoules either in a cotton wool or in a paper. The semen transported at 5 °C is packed in appropriate isolated Styrofoam containers that are fitted either with frozen water bottles, frozen gel packs, or crushed ice. The direct contact between the semen and the ice is usually avoided by a layer of uncooled thermal gel that serves as a shock absorber and thermal conveyor.
The sperm sensitivity to lower temperatures is likely associated with a higher unsaturated to saturated fatty acid ratio in the plasma membrane (
36). Consequently, the species can be divided into two classes: species that either have higher or lower content of unsaturated fatty acids in the sperm membrane. The bull, ram, and boar sperm have higher susceptibility to cryodamage due to the higher unsaturated to saturated acids ratio (>2.5), whereas rabbits, dogs, and humans belong to a more resistant sperm group (having lower ratio of about 1.0) (
37). Our previous studies have concurrently indicated that ram ejaculates with higher sperm motility contained lower level of poly-unsaturated fatty acids (PUFA). The peroxidation processes occurring almost regularly in all
in vitro conditions are affecting PUFA molecules resulting in release of oxygen-reactive-species (ROS) which in turn, cause chain progression of the peroxidation to other PUFA molecules (
38).
Due to the high PUFA content of sperm cells in almost all domestic species, they are highly prone to lipid peroxidation compared to somatic cells. High levels of plasma membrane lipid peroxidation largely affect the membrane stability leading to compromised integrity and eventually to cell death. Furthermore, ROS can impair sperm motility and chromatin integrity resulting in decreased fertile capacity of the semen (
39). Numerous researchers have been focusing on the effects of different antioxidants supplemented to sperm cryopreserving media (oxidized glutathione-GSSG, reduced glutathione-GSH, cysteine) in aim to reduce the oxidative stress when supplemented in ram semenfreezing media. Most of these studies reported that these antioxidants have no effect in increasing ram sperm viability during cryopreservation (
40, 41).
Insemination methodsOne of the most obvious limiting factors of successful AI and CR is the
in utero placement of the ejaculate due to the specific anatomy of the ewe’s cervix (
42, 43). Several approaches have been suggested for overcoming of this issue. The first suggested approach was forceps retraction of the cervix into the vagina which enables higher visual field of the cervical opening thus enabling easier insertion of the inseminating instrument through the cervical canal (
42, 44). The second approach utilized pharmaceutical treatments initiating cervical relaxation and dilation: PGE
2 or PGEanalogues, oxytocin, interleukin-8, misoprostol, hyaluronic acid (
10, 45), and beta-adrenergic blocker - carazolol (
46). The third approach utilized catheters for trans-cervical AI and ET (
10).
Additionally, the Guelph insemination method was modified to provide transcervical semen deposition resulting in higher pregnancy rate compared to the vaginal and cervical insemination methods. Furthermore, other studies have used the transcervical AI (
10) following PGE
2 (
47) or PGE
1 analogues (
48) treatments achieving successful cervical penetration but without a significant increase in the lambing rate. When using frozenthawed semen for AI, higher fertility rates were achieved by intracornual insemination using laparoscopic technique (
Fig. 3) (
39, 49, 50). The sperm is directly deposited into the top of the uterine horn, thereby avoiding sperm loss during its passage through the cervical canal, uterine body, and the caudal part of the uterine horns. Several studies have utilized this method in small ruminants reporting various AI success rates (
51, 52, 53).
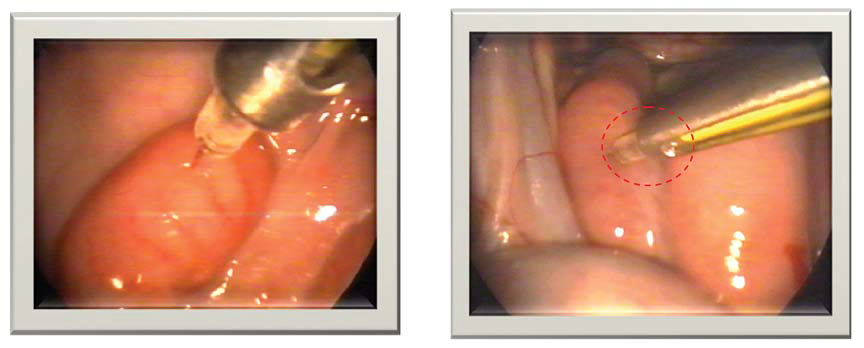 Figure 3.
Figure 3. Correct (left) and incorrect (right) intra-cornual laparoscopic insemination in sheep (
48)
Cervical dilation methodsDinoprostone (10 mg) is used as vaginal insert in human gynaecology for induction of cervical ripening and labour in women. It contains 241 mg hexanetriol, macrogol 8000, isocyanate crosslinked hydrogel matrix copolymer, and 10 mg of dinoprostone. A Canadian study conducted on Rideau Arcott sheep breeds reported that the dinoprostone vaginal insert facilitated the sperm deposition into the uterine body by using the Guelph insemination technique but has not increased the pregnancy rate (
47). Misoprostol is a synthetic analogue of prostaglandin E
1 (PGE
1) that acts as a cervical dilatant. Its effects are usually manifested 24-72 hours following the treatment with maximal cervical dilation following 54 h (
48). However, the pregnancy rates were not reported in this study. Another study reported the use of human interleukin 8 (HuIL-8) for cervical dilation in ewes (
54). This study reported that HuIL-8 increased the neutrophils and collagenase in the cervix during the early postpartum period in mammals but did not cause cervical dilatation. Another study utilized exogenous oxytocin for cervical dilatation reporting higher success in insertion of the inseminating catheter (
55). Although, the treatment with exogenous oxytocin causes a higher rate of cervical dilatation facilitating successful semen deposition in the uterus, an improved fertilization and lambing rate have not been observed following the transcervical insemination (
56).
IN VITRO EMBRYO PRODUCTION
In vitro maturation (IVM)The
in vitro maturation (IVM) of oocytes includes alterations of both the cytoplasm and nucleus in a synchronous manner. The oocyte heterogeneity in quality can be a source of high variability in IVM maturation but in most instances the success in reaching metaphase II can be higher than 80% in sheep (57, 58). Oocytes are conventionally placed in IVM medium in groups of 25-50 and incubated at 38-39 °C for 24-27 hours in 5% CO
2 atmosphere (
59).
The TCM199 is one of the most frequently used IVM media which contains bicarbonate buffer, antioxidants, minerals, vitamins, glucose, glutamine, amino acids, pyruvate, FSH, LH, estradiol, heattreated serum, and follicular fluid from non-atretic follicles (
60). The thiol-groups of antioxidants (glutathione-GSH, cysteine, and cysteamine) reduce the ROS which are produced in the 5% CO
2 incubation conditions and increase the intracytoplasmic concentrations of GSH (
61). The TCM199 contains thiamine hydrochloride, riboflavin, pyridoxal hydrochloride, folic acid, d-calcium pantothenate, nicotinamide, myoinositol, and choline chloride. The combined use of minimal essential medium (MEM), containing the same vitamins as TCM199, synthetic oviductal medium (SOF), and bovine serum albumin (BSA) was reported to increase the blastocyst development in sheep (
62).
The gonadotropins and 17β-estradiol contained in the IVM media (LH 5 μg/ml, FSH 5 μg/ml, and 1 μg/ml 17β-estradiol) (
63) are used for increased oocyte maturation and expansion of cumulus cells (
64).
Human (hCG) and equine chorion gonadotropins (eCG) are also reported in IVM media for sheep (
62). Serum and follicular fluid composition tends to be highly variable and therefore, is reported to constitute between 10 and 20% of the IVM media. These fluids are a source of proteins and growth factors which facilitate the process of maturation and subsequent development (
59).
The following sera are reported for use in IVM media for sheep: fetal calf serum (
65), estrus goat and estrus sheep serum (
56). Follicular fluid collected from follicles >4 mm was reported to have beneficial effects on the maturation of sheep oocytes (
66), but it must be considered that high variations in their composition could be a source of high variability in different reports (
59).
Epidermal growth factor (EGF) is frequently used in sheep IVM media (
62). The combined use of TC199, cysteamine, and EGF was reported to increase the maturation in sheep oocytes (
67). The effect of insulin-like growth factor-I (IGF-1), EGF, cysteamine, was compared in defined, semidefined, and undefined IVM medium for sheep oocytes reporting a higher percentage of morula and blastocysts in the undefined IVM media (
68).
The addition of growth hormone (GH) (300 ng/ml) and serum in IVM media has been reported to yield higher percentage of cleavage, blastocysts, and hatching (
69). This effect was not observed when serum was not added.
In conclusion, sheep oocytes recovered from non-atretic follicles >3 mm can be matured in semi-defined or defined IVM media with addition of EGF and cysteamine. A study of Souza-Fabjan et al. 2014 (
70) demonstrated that there were no significant differences in blastocyst production between different compositions of IVM media: 1. TCM199, cysteamine, and EGF; 2. TCM199 and fetal calf serum; and 3. TCM199, serum, hormones, vitamins, antioxidants, EGF, and IGF-1. If prepubertal oocytes are used, a more complex undefined IVM media are required (TCM199, antioxidants, serum, and hormones) (
71).
In vitro fertilization (IVF)Sperm selection is one of the most crucial steps in successful
in vitro fertilization (IVF) since ejaculates are a mixture of mature, immature, viable, non-viable spermatozoa, and other type of non-reproductive cells. The swim-up procedure is frequently reported as a method for separating the motile from non-motile sperm in sheep (
65, 72). An aliquot of fresh sperm (50-100 μl) is placed under a volume of capacitating medium (2 ml) in several tubes. Following 0.5-1-hour incubation at 38.5 °C, the upper level of the medium (0.5-1 ml) is collected and centrifuged (200 x g) for 10 minutes. The Percoll gradient method is used for frozenthawed ram sperm (
73, 74). The Percoll granules are composed of colloidal silica (15-30 nm) coated with polyvinylpyrrolidone. The gradient is formed by adding 1.5 ml of 90% Percoll solution at the bottom of a conical tube, overlayered with another (1.5 ml) 45% Percoll solution. The thawed semen is placed on the top layer and centrifuged (600-1000 x g) for 10-15 minutes at room temperature. By removing the supernatant, the pellet is collected and washed twice by centrifugation and resuspension in 0.5-2 ml of medium. It was reported that the Percoll gradient method achieved significantly (P<0.05) higher cleavage and blastocyst formation than the swim-up method in bucks (62% and 18% vs. 50% and 11%, respectively) (
75).
Sperm capacitation is performed
in vitro by using estrus sheep serum (
76), heparin (
77), heparin and inomycin (
78), heparin, penicillamine, hypotaurine and epinephrin (
79), heparin and serum (
80), and heparin, serum, penicillamine, hypotaurine and epinephrin (
81). Sperm can be incubated (15-60 min) with these additives either prior or during the IVF. The estrus sheep serum has been reported to yield significantly higher cleavage rate of sheep oocytes in the IVF medium compared to BSA (78% and 0%, respectively) (
82). The syntenic oviductal fluid is the most frequently used IVF media (
63, 74).
Oocytes (15-30) are placed in an IVF medium drop and fertilized with an average sperm concentration of 1x106/ml. The incubation of both gametes is performed at 38-39 °C for 16-24 hours, and humidified air with 5% CO
2 (83).
In vitro embryo cultureThe percentage of blastocysts developed from fertilized oocytes may vary depending on media composition, pH, temperature, CO2, oxygen, growth factors, and cytokines (
59). The early embryos up until the 8- to 16-cell stage have more stable metabolic activity and are more easily sustainable. Passing this stage, they undergo genomic activation and increased metabolic activity rendering them unstable for
in vitro conditions (
59). By using oviductal epithelial cells it is possible to attain higher percentage of embryos passing the 8- to 16-cell stage (
75, 84). Oviductal epithelial cells used in a culture medium yielded significantly higher blastocyst percentage compared to cumulus cells (
85).
To reduce the ROS production in 20% oxygen atmosphere, alpha-tocopherol or L-ascorbic acid cold be used. Blastocyst yield in sheep was reported 18% vs 8% and 14% vs 9% with or without use of the respective antioxidants (
86, 87).
The use of serum in the medium might trigger ‘large offspring syndrome’ in lambs which may include large and disproportionate fetus development, altered organ morphology, and placental abnormalities (
65). The period in which the embryo is dependent from maternal RNA through the time of the major genome activation is considered as the most sensitive period to environmental stress which could trigger the 'large offspring syndrome' irrespective whether serum was used or not in the medium (
88).
There are several different
in vitro culture mediums used and reported in the literature: TCM199 (
65), Sydney IVF Blastocyst (
84, 89) and B2 (
84). The SOF is most frequently reported with 10% fetal calf serum (
63) or BSA (
74). Cox and Alfaro (
80) have reported 61.5% blastocyst rate in sheep oocytes recovered by laparoscopy and cultured in SOF and BSA for 5 days, and in TCM199 for 2 more days (
80). The addition of ghrelin (50 ng/ml) in the SOF medium has increased the blastocyst rate, cell number, and the expression level of GLUT1 and IFNT genes (
74). Receptors for activin have been localized in oocyte and granulosa cells in various developmental stages in ovine follicles (
90). Hence, the addition of activin-A (10 ng/ml) has increased the development in oocytes of prepubertal goats (
91).
The sequential media have been introduced as a way to mimic the natural changes in the environment of the developing embryo (
92). Its composition changes throughout different stages of the development in order to meet the requirements and decrease the stress to the embryo (
93). In sheep, the sequential media G1.3/G2.3 supplemented in BSA have obtained 21.5% blastocyst rate compared to the culturing in SOF plus 5% fetal calf serum (
94). However, the hatching rate was 44.3% and 86.6%, respectively.
Personal clinical experience in assisted reproduction methodsTwo experiments were conducted aiming to test the
in vivo fertility rate of frozen-thawed ram semen from the ovchepolean pramenka breed (
49).
In the first experiment, 86 ewes were synchronized by progesterone-based protocol and subsequently inseminated using either intracervical (n = 42) or intrauterine (n = 44) method. Inseminations were performed at 50-52 h (intracervical) and at 52-54 h (intrauterine) after withdrawal of the vaginal progesterone inserts (FGA 20 mg), with concomitant ECG application (500 IU). A French insemination gun for small ruminants was used for the intracervical insemination inserting semen volume of 0.5 ml (150-200x106 spermatozoa). The laparoscopic AI (LAI) was carried out using a Karl-Storz equipment set and Robertson semen application pipette, inserting a dose of 0.25 ml applied directly in the lumen of the uterine horns. The pregnancy diagnosis was performed at day 60 following AI by ultrasound. The results have shown a satisfactory overall fertilization rate of 48.84%. Additionally, the fertility rate was significantly higher (P<0.05) in ewes inseminated by intrauterine application (54.54%) in comparison to the ewes inseminated by intracervical method (42.86%). These results were in agreement with our previous report (
95) in which fertility rates were 54%, 52%, and 38% for unicornual, bicornual LAI, and cervical insemination, respectively.
In the second experiment, seven ovchepolean pramenka ewes were inseminated by LAI after superovulatory oestrus induced either by 1200 IU ECG - Group 1 (n=4) or by 200 mg NIH-FSH-P1 Group 2 (n=3). The superovulatory protocol included 6 injections in each group, administrated at 12 h intervals in decreasing doses (50+50 mg, 30+30 mg, 20+20 mg), starting at day 10 after the progesterone treatment. LAI was performed as described in Experiment 1, except that the Robertson pipette designed for ovine donors was not used. Seven days later, the embryos were surgically recovered by flushing both uterine horns and oviducts. A lower superovulatory response was observed in both groups which was in accordance to the number of existing
corpus luteum on the ovaries: 9 in Group 1 (2.3 per donor) and 12 (4 per donor) in Group 2. In addition, six embryos plus one unfertilized oocyte, and five embryos plus two unfertilized oocytes were obtained from Group 1 and Group 2, respectively, with uterine flushing rate of 77.78% for Group 1 and 58.33%, for Group 2. Nevertheless, the conception rate in both groups reached a satisfactory level of 83.33% and 71.43% in Group 1 and Group 2, respectively (
49).
SEX SORTED SEMEN
The most efficient way to shift the offspring sex ratio is by using sex-sorted semen (
94). The use of ram sex-sorted semen has been less frequently reported than in other animal species. The success in fertilization by using ram sex-sorted semen is highly affected by the spermatozoa concentration. In a study by Hollinshead et al. (
97), it was reported that by using sex-sorted and cryopreserved ram spermatozoa the pregnancy rate was 25% and 15% for X and Y-chromosome, respectively, which was significantly lower compared to the control 54%. The authors concluded that the lower fertility in one of the rams affected the pregnancy rates in the ewes but suggested that the minimum frozenthawed spermatozoa concentration for successful intrauterine fertilization when using sex-sorted semen is 2-4x10
6 motile cells (
97).
The method of flow cytometry has high efficiency in separating the spermatozoa according to their sex chromosome’s structure. Although, this method has been already commercialized in the cattle industry, its implementation for ram semen is still in its initial phase. Unlike other domestic species, ram spermatozoa showed higher structural resistance when submitted to the sex sorting process. It was reported that cryopreserved sexed ram spermatozoa had higher motility, viability, acrosome integrity, and mitochondrial activity, but reduced velocity and cervical mucus penetration compared to the non-sexed cryopreserved spermatozoa (
98). The flow cytometry might be selecting spermatozoa by both sex chromosomes and higher viability which could explain the previous report. Higher fertility rate was reported in the ewes inseminated with sexed frozen/thawed semen compared to those inseminated with non-sexed semen (
99). Therefore, these results might encourage researchers to focus on further development of this method.
CONCLUSION
AV is the most plausible and convenient method for semen collection in rams but is limited to reproductive centers. EE could be used in rams which are not routinely sampled by AV and would need training. Direct semen collection from the epididymis is useful in rams that would be castrated or used for single sampling. Despite the classical semen assessment methods (volume, consistency, density, color, pH, CASA kinetic parameters, supravital staining, HOS), fluorescent dyes (Sybr14 and propodium iodide) are routinely used for assessment of plasma and acrosome membrane integrity. Sperm Chromatin Structure Assay is complementary semen assessment method, which detects potential chromatin decondensation. For highly valued rams, sperm proteomics offers detection of high-fertility biomarkers. For example, PEBP4, SPATA18, and CPVL are biomarkers for high sperm motility, NUP98 is associated with lower apoptotic rate, and CPVL, IZUMO1, and AGA are associated with increased sperm-egg fusion. Additionally, hamster zona-free ovum test and the hemi-zona test could be used to assess the capacitation, acrosome reaction, eggfusion, and sperm chromatin decondensation in sperm cells. The use of frozen-thawed ram semen and its fertility is still an ongoing topic in small ruminant reproduction due to its significantly lower fertility compared to chilled semen. The Guelph insemination method has been reported to yield higher pregnancy rates compared to the vaginal and cervical insemination methods. TCM199 is one of the most frequently used IVM media. Sheep oocytes recovered from non-atretic follicles >3 mm can be matured in semi-defined or defined IVM media with addition of EGF, and cysteamine. Oviductal epithelial cells used in a culture medium yielded significantly higher blastocyst percentage compared to cumulus cells. The addition of ghrelin in the culture medium could produce higher blastocyst rate, cell number, and higher expression level of GLUT1 and IFNT genes. Sequential media are employed as a novel culture method which meets the embryo requirements in various stages in its development. Sex-sorted semen could be utilized for planned sex ratio in the herd but its success is highly dependent on the sperm concentration in a dose since its fertility is significantly lower than the non-sexed semen. An overall improvement of the farm management and production systems needs to be accomplished as a prerequisite for successful implementation of these AI methods. This in turn should demonstrate the synergy between farmers profit and AI.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Faculty of Veterinary Medicine – Skopje, Ss. Cyril and Methodius University in Skopje and the Faculty of Veterinary Medicine in Zagreb, University of Zagreb, for sharing their logistical, intellectual, and research infrastructure which was used to convey the experimental, statistical and analytical work for this study.
AUTHORS’ CONTRIBUTION
TD conceived and designed the study and wrote the paper; PT performed laparoscopic intrauterine insemination in ewes, performed the flushing of embryos and wrote the subchapter ‘Embryo Transfer’; BA performed the ovine intracervical insemination with frozen ram semen and performed the semen collection from rams; MN performed the statistical analysis and wrote the subchapters ‘Semen Assessment’, ‘Semen Conservation/Preservation’ and ‘Sex Sorted Semen’; FPP wrote the subchapter ‘In Vitro Embryo Production’; MD contributed data or analysis tools and wrote the subchapters ‘In Vitro Maturation and In vitro Fertilization of Oocytes’; VP collected data from the semen evaluation and in vivo experiments for assessment of ram frozen semen and wrote the subchapter ‘Microscopic Ram Semen Analysis’; JG collected data and wrote the subchapter ‘Semen Collection and AI’; SV performed the statistical analysis from semen evaluation and Artificial Insemination.

 10.2478/macvetrev-2022-0018
10.2478/macvetrev-2022-0018


