Acute diarrhea (AD) has a complex etiology and may lead to life-threatening conditions. Hematological and serum biochemistry analyses can be useful for a differential diagnosis and for determining the severity of diarrhoea. Dogs with AD (n=72) were divided into Isospora (n=18), Toxocara (n=18), Parvoviral Enteritis (n=18), and Dietary Diarrhea (n=18) subgroups following clinical and laboratory examinations. The study aimed to evaluate the diagnostic value of certain hematological and serum biochemistry parameters. Clinical examinations, rapid diagnostic tests, complete blood count (CBC), and biochemical analyses were performed. White blood cell count (WBC), granulocyte, and mean hemoglobin concentration (MCH) levels were lower in the Parvoviral Enteritis Group compared with the other groups (p<0.01). Isospora, Parvoviral Enteritis, and Toxocara groups had lower glucose and total protein, and higher creatinine levels than those of the Control and Dietary Diarrhea groups (p<0.0001). The albumin level of the Dietary Diarrhea Group was higher compared with the other groups (p<0.0001). Parvoviral Enteritis and Isospora groups had higher ALP levels than those of the other groups (p<0.0001). Blood urea nitrogen (BUN), alanine aminotransferase (ALT), C-reactive protein (CRP), and cholesterol levels were determined to be highest in the Parvoviral Enteritis Group (p<0.0001). The total bilirubin level was higher in Parvoviral Enteritis and Toxocara groups compared with the Control, Isospora, and Dietary Diarrhea groups (p<0.0001). As a result, it was concluded that in cases of AD due to parvoviral enteritis and Toxocara canis, serum biochemistry abnormalities may be more severe, can provide more clinical information than CBC, and can be useful in forming a differential diagnosis list, especially in triage.
Among the etiology of canine diarrhea,
Toxocara canis is an important endoparasite causing acute diarrhea (AD), especially in shelters with poor hygiene conditions. Clinical symptoms due to
Toxocara canis are weight loss, anemia, vomiting, intermittent diarrhea, neurological symptoms, and even death due to intestinal obstruction (
3). Another important endoparasite in the etiology of canine diarrhea is
Isospora spp. Clinical symptoms such as vomiting, abdominal pain, and watery acute diarrhea occur mostly in young, weak, and immunosuppressed animals (
4). One of the most common viral agents of acute (CPE) which is a highly contagious, fatal disease (
5). Typical findings in CPE are vomiting, severe hemorrhagic diarrhea, and immunosuppression (
6). Many bacterial pathogens may play a role in dogs’ bacterial enteritis. Commensal bacterial increase and dysbiosis can cause bacterial or dietary diarrhea. Limited understanding of the nature and complexity of the intestinal flora and lack of specific diagnostic tests limit the ability to identify a broader range of pathogens on a routine basis (
7).
Laboratory confirmation of the causative agent(s) of diarrhea in puppies may allow for appropriate treatment. Even though hematochemical parameters are not sufficient to identify the exact cause of AD, they are useful in forming a differential diagnosis list. Irrespective of etiology, AD leads to electrolyte imbalance and dehydration (
8). Significant loss of intravascular volume resulting in dehydration may lead to hemodynamic instability, decreased tissue perfusion, cellular hypoxia, hypertension, organ damage, and even death (
9). Thus, evaluation of certain clinical, hematological, and serum biochemistry parameters indicating the status of vital organs allows early therapeutic intervention and may prevent target organ damage. Therefore, the aim of this study was to evaluate the hematological and serum biochemistry parameters and to reveal their diagnostic importance in dogs with AD due to non-infectious and/or infectious etiologies.
MATERIAL AND METHODS
This study was conducted with the approval of the Harran University Animal Experiments Local Ethics Committee dated 16/12/2021, number 2021/009.
Animal selectionThis study included 72 dogs with diarrhea (Diarrhea Group) and 16 healthy dogs without any complaints (Control Group) which were brought either for diagnosis/treatment or vaccination/ routine check-up purposes between January 2021 and December 2021 to Konya Selcuk University and Şanlıurfa Harran University Animals hospitals of Veterinary Faculties. All dogs were owned at an age lower than six months, were unvaccinated, mixed breed, and were fed on commercial dry dog food. The owners reported that the dogs in the Diarrhea Group had diarrhea for less than 6 days and no treatment was given for this condition. The presence of active diarrhea less than 7 days and weighing 4-10 kg were accepted as inclusion criteria. Complete anorexia, dehydration >10%, respiratory, endocrine, renal or dermatological problems were exclusion criteria according to the previous reports for AD cases (
2).
Clinical examinationsBody temperature, respiratory and pulse rates, capillary refill time (CRT), lung and heart auscultations, and palpable lymph nodes evaluations were performed. In addition, microscopic fecal examinations of all dogs were performed with an appropriate method (zinc sulfate centrifugal flotation method for
Isospora spp., centrifugal flotation method with a solution of sodium nitrate (NaNO
3, SG=1.32) for
Toxocara canis and other parasite eggs) (
10).
Blood samplingVenous blood samples (4-6 ml) were obtained via vena cephalica venipuncture with minimal patient stress. After sampling, a portion of the collected blood samples was immediately transferred into tubes containing Na EDTA for CBC analysis. Another portion was transferred into tubes without anticoagulant for serum biochemistry analysis. The blood samples in the tubes without anticoagulant were centrifuged at 5000 rpm for 5 minutes at room temperature for serum extraction.
Complete blood count analysisWithin the scope of CBC analysis, white blood cell (WBC), lymphocyte, monocyte, granulocyte, red blood cell (RBC), mean corpuscular volume (MCV), hematocrit, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and hemoglobin levels were measured using a hematology auto analyzer (pocH-100i®, Sysmex, Kobe, Japan) in Harran University Central Laboratory of Veterinary Faculty.
Serum biochemistry analysisSerum glucose, creatinine, cholesterol, alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, albumin, total protein, C-reactive protein (CRP) and blood urea nitrogen (BUN) levels were measured using an automatic biochemistry analyzer (Spotchem EZ SP 4430®, Arkray Inc., Kyoto, Japan) within the scope of serum biochemistry analysis in Harran University Central Laboratory of Veterinary Faculty.
Forming subgroupsFecal samples were taken with sterile swabs directly from the rectum to subdivide the Diarrhea Group on an etiological basis. Microscopic fecal examination (CX43 light microscope, Olympus®, Tokyo, Japan) was performed with zinc sulfate centrifugal flotation and centrifugal flotation with a solution of sodium nitrate methods. In addition, rapid diagnostic tests for
Canine Parvovirus/Canine Coronavirus/Giardia spp. (CPV/CCV/Giardia Ag, Anigen Rapid®, Bionote, Gyeoggi-do, Korea). The sensitivity and specificity were determined as follows: for CPV subtypes (CPV2, 2a, 2b, 2c) - sensitivity: 100%, specificity: 98.8%; for CCV - sensitivity: 93.1%, specificity: 97.5%. The detection limit for Giardia was 125/100 μl. According to the microscopic fecal examination and rapid diagnostic test results, the Diarrhea Group was divided into Isospora (n=18), Parvoviral Enteritis (n=18), and Toxocara (n=18) groups. Dogs with no parasites/parasite eggs in the microscopic fecal examination, negative rapid diagnostic test results, mild leukocytosis, and the dietary change recorded by anamnestic data were included in the Dietary Diarrhea (n=18) group. The same tests and examinations were also applied to the dogs of the Control Group and they were all determined to be negative and/or within reference ranges. In addition, no recent diet change was confirmed by anamnesis.
Statistical analysisData analysis was performed using statistical software (SPSS 25.00, IBM®). In order to determine whether the variables had normal distributions, the one-sample Kolmogorov-Smirnov test was used. Parametric data were evaluated by Student t-test and presented as mean ± standard error of mean (SEM). The one-way analysis of variance (ANOVA) was used to determine whether there were significant differences between the groups. The statistical significance level was p<0.05.
RESULTS
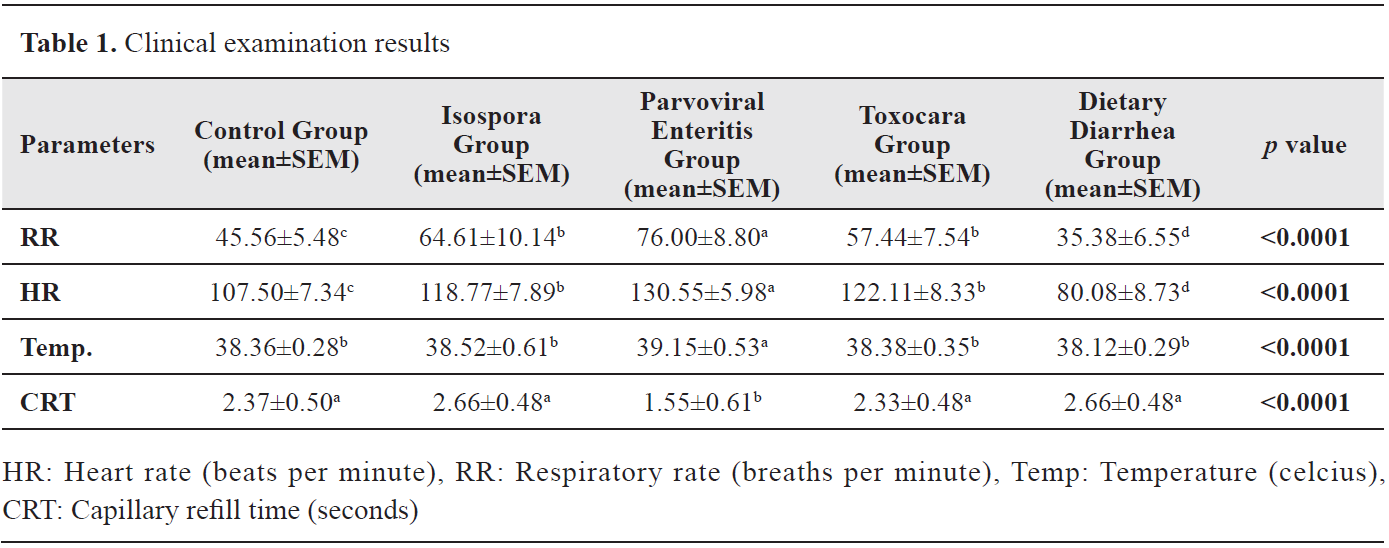
Clinical examination resultsStatistically significant differences were observed in the values of body temperature, CRT, pulse rate (HR), and respiratory rate (RR) (p<0.05). The RR and HR of the Parvoviral Enteritis Group were determined to be higher compared with the other groups (p<0.0001) while no differences were observed between the Isospora and Toxocara groups. The body temperature value of the Parvoviral Enteritis Group was significantly higher compared with the other groups (p<0.0001). In addition, the CRT value was determined to be the lowest in the Parvoviral Enteritis Group (p<0.0001). Clinical examination results are presented in
Table 1.
 Complete blood count analysis results
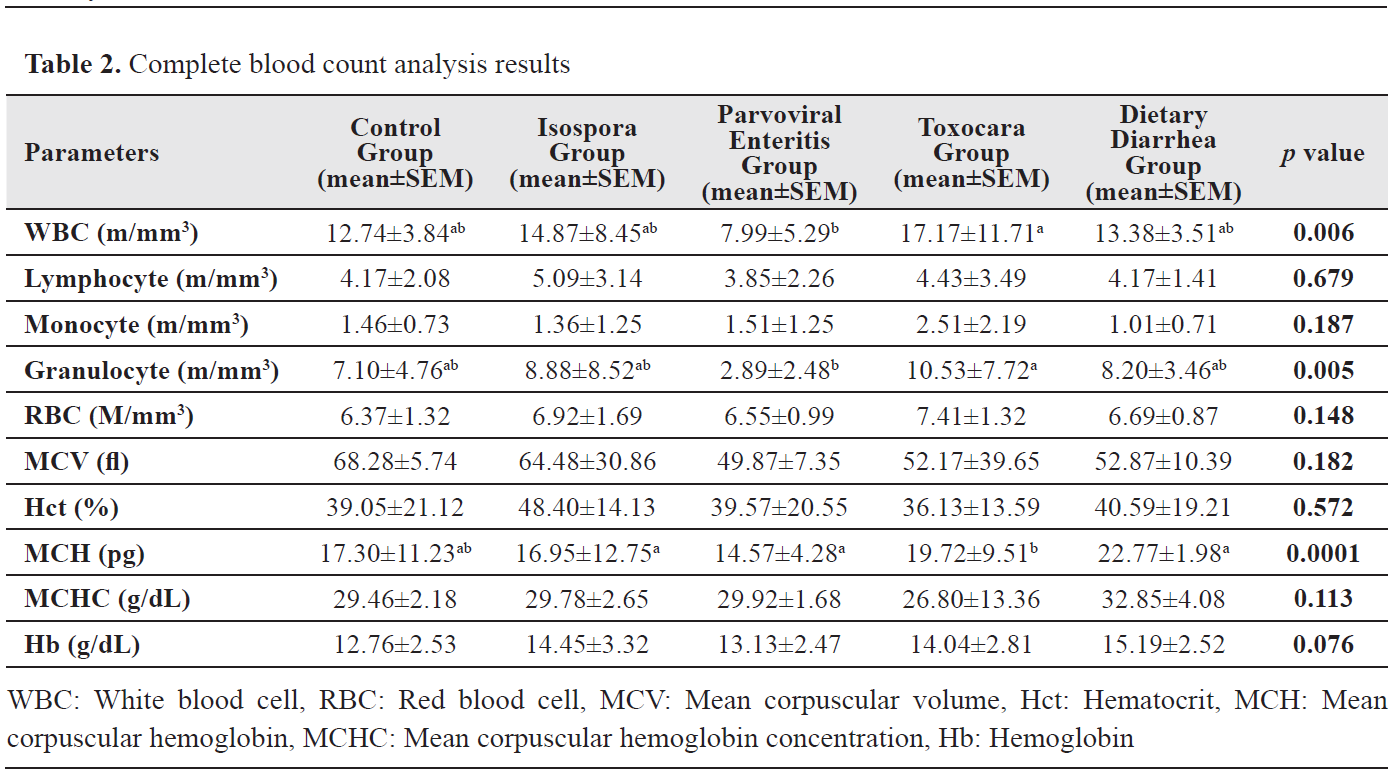
Complete blood count analysis resultsSignificant differences were found in WBC, granulocyte, and MCH levels (p<0.05). A significant difference was observed between the Parvoviral Enteritis and Toxocara groups in WBC and granulocyte levels with the lowest value observed in the Parvoviral Enteritis Group (p<0.006). MCH level was significantly different in the Toxocara Group when compared with Isospora, Parvoviral Enteritis, and Dietary Diarrhea groups (p<0.0001). CBC analysis results are presented in
Table 2.
 Serum biochemistry analysis results
Serum biochemistry analysis resultsWhile no differences were determined in serum glucose levels between the Control and Dietary Diarrhea groups, they were higher than those of the Isospora, Parvoviral Enteritis, and Toxocara groups (p<0.0001). The creatinine level of the Toxocara Group was significantly different compared with the other groups (p<0.0001). Cholesterol level was the highest in the Parvoviral Enteritis Group (p<0.0001). The ALT level was lower in the Isospora Group than those of the other groups (p<0.0001). In addition, the ALT level of the Parvoviral Enteritis Group was higher than the Control and Dietary Diarrhea groups (p<0.0001). There was a significant difference between the Parvoviral Enteritis and Isospora groups for the ALP level (p<0.0001). The total bilirubin level was higher in Parvoviral Enteritis and Toxocara groups compared with the Control, Isospora, and Dietary Diarrhea groups (p<0.0001). Albumin level was higher in the Control and Dietary Diarrhea groups compared with the other groups (p<0.0001). Total protein levels of the Parvoviral Enteritis, Isospora, and Toxocara groups were lower than those of the Control and Dietary Diarrhea groups (p<0.0001). The CRP level was the highest in the Parvoviral Enteritis Group (p<0.0001). The BUN level was significantly different between the Toxocara, Isospora, and Parvoviral Enteritis groups with the highest value in the latter group (p<0.0001). Serum biochemistry analysis results are presented in
Table 3.

DISCUSSION
Diarrhea is considered acute if it lasts less than 7 days (
2, 11). Even though its etiology is complex, diarrhea may occur due to infectious diseases as well as due to dietary changes (
2). Most AD cases, such as dietary diarrhea, are mild and self-limiting and likely associated with changes to the intestinal microbiota. However, a thorough and logical diagnostic plan must be followed to obtain an accurate diagnosis and guide appropriate therapy (
12). In this study, significant clinical, hematological, and serum biochemistry alterations, which can be used to assess the severity of the clinical manifestation, were determined in dogs with AD due to different etiologies.
Clinical symptoms such as anorexia, weight loss, abdominal pain, vomiting, watery to hemorrhagic diarrhea, mild fever, and prolonged or shortened CRT which may indicate hyperdynamic status as a result of pain, fever, and/or infection and dehydration have been reported in dogs with AD (
5). Along with these non-specific clinical symptoms, findings related to systemic inflammatory response syndrome (SIRS), especially in AD due to viral agents such as CPV, can be observed (
13). As a result of the clinical examinations in the current study, the shortest CRT, the highest body temperature, RR, and HR values were observed in the Parvoviral Enteritis Group (p<0.0001). There were no differences in body temperature and CRT values in the Dietary Diarrhea, Toxocara, and Isospora groups compared with the Control Group. Also, RR and HR values of all dogs with AD were higher when compared with the Control Group (p<0.0001). These findings may indicate that diseased dogs develop mild to moderate diarrhea-related dehydration, which may result in tachycardia and poor peripheral perfusion (
12). Also, it was thought that septicemia/endotoxemia or SIRS findings due to bacterial translocation in CPE cases could be more severe than the diarrhea cases due to endoparasite and/or dietary changes (
5).
Leukopenia is the prominent hematological abnormality in CPE due to the destruction of bone marrow precursors and the increased demands of the massively inflamed intestinal tract. Anemia, thrombocytosis, and neutrophilic leucocytosis are also reported (
12). Accordingly, lower WBC and granulocyte levels of the Parvoviral Enteritis Group (p<0.01) as a result of CBC analysis were thought to be associated with the destruction of bone marrow precursors and the depletion of lymphoid tissues (
5). As there were no differences in WBC and granulocyte counts in the Isospora and Toxocara groups compared with the Control Group, these findings may be related to the fact that these endoparasites cause immunosuppression only in chronic cases (
9). In addition, the normal CBC findings of the Dietary Diarrhea Group may be related to the fact that diarrhea is only due to mild intestinal dysbiosis (
2, 12). There was no difference in the MCH levels between the Control and the other groups. The Toxocara Group was significantly different compared to the other groups except for the Control (p<0.0001). This finding was interpreted as an invasion with
Toxocara canis resulting in lowered absorption capacity and affected intestinal mucous membrane (
14). The other CBC parameters which were not significantly different and were within the reference ranges may be related to the course of the diseases and the time of admission to the hospital (
15).
Juvenile dogs have limited glycogen stores and scarce hepatic gluconeogenic activity requiring oral intake to maintain blood glucose concentrations which can be compromised by malnutrition due to vomiting, diarrhea, and anorexia (
13). For this reason, hypoglycemia is considered as severe complication of enteritis. Additional causes of hypoglycemia include sepsis and liver dysfunction (
16). Low serum glucose levels of the Isospora, Parvoviral Enteritis, and Toxocara groups compared with the Control and Dietary Diarrhea groups (p<0.0001) may be associated with increased glucose demand, anorexia, liver dysfunction as well as glucose consumption by bacteria, neutrophils, and peripheral tissues (
17) in cases of Isosporiasis, Toxocariasis, and Parvoviral enteritis (
18).
It has been reported that serum BUN and creatinine levels increase as a result of dehydration due to diarrhea in AD cases (
9). In the present study, the higher BUN and creatinine levels of the Isospora, Parvoviral Enteritis, and Toxocara groups compared with the Control and Dietary Diarrhea groups (p<0.0001) were thought to be associated with higher fluid loss and tissue hypoperfusion (
16). In addition, the highest BUN level in the Parvoviral Enteritis Group among all the other groups was associated with the presence of more severe malabsorption and fluid loss due to villous atrophy in cases of CPE (
19). Moreover, although serum BUN and creatinine levels are non-specific markers for early renal damage, high BUN and creatinine levels of the Isospora, Parvoviral Enteritis, and Toxocara groups may be associated with acute kidney injury (AKI) in addition to dehydration (
20). The lack of difference between the Dietary Diarrhea and the Control groups was thought to be related to the fact that diet-induced diarrhea develops as a result of dysbiosis (
12).
Low cholesterol levels have been reported in dogs with critical diseases such as parvoviral enteritis. Contrary to previous reports (
21), in the present study, it was observed that the highest cholesterol level was in the Parvoviral Enteritis Group compared with the other groups (p<0.0001). This finding may be related to increased lipoprotein production and/or decreased lipoprotein clearance as a defense mechanism by the host (
22).
As in dogs with CPE (
23), high ALT and ALP levels have been reported in dogs with Toxocariasis (
24), which is mainly due to the migration of
Toxocara canis through the liver parenchyma (
14). It was reported that the agent can be seen in the Kupffer cells of the liver and macrophages in the portal areas, and also moderate steatosis and cholestasis may develop in cases of Isosporiasis (
25). In the present study, when compared with the Control and Dietary Diarrhea groups, the higher ALT level of the Parvoviral Enteritis Group may be associated with dehydration and severe hypoperfusion (
26). In addition, higher ALP levels of the Isospora, Parvoviral Enteritis, and Toxocara groups compared with the Control and Dietary Diarrhea groups may be related to the damage and/or necrosis of intestinal tissue (
3). Moreover, in cases of severe infestation, the excessive elevation of these enzyme levels was reported to be associated with increased permeability of hepatocytes and intestinal tissue allowing more liver enzymes to diffuse into the bloodstream as well as insufficient tissue perfusion (
18).
Bilirubin has exhibited anti-inflammatory and antioxidative activities in various pathologies by reducing the inflammatory factor products and oxidative stress. Thus, the induced synthesis of bilirubin can counteract oxidative stress (
27). In addition, several studies have reported that bilirubin exerts anti-inflammatory effects (
28). In the present study, the higher total bilirubin levels of the Parvoviral Enteritis and Toxocara groups compared with all the other groups may be associated with the higher pro-inflammatory cytokine release and higher inflammation response in cases of CPE and Toxocariasis (
13, 16, 27).
It has been reported that a serum albumin concentration of less than 2 g/dL is an indicator of poor prognosis in dogs with inflammatory bowel disease due to protein loss (
29). Also, it was reported that protein-losing enteropathy may develop in cases of AD due to intestinal villus damage, especially in puppies (
3). In the present study, the serum albumin levels of the Isospora, Toxocara, and Parvoviral Enteritis groups were lower when compared with the Control and Dietary Diarrhea groups (p<0.0001). This finding may be related to the fact that more severe mucosal damage develops and more protein is lost in secretory diarrhea than in osmotic diarrhea due to dietary changes (
12). Moreover, negative acute phase response to the inflammatory process may support this finding (
30). Since the albumin levels of the Isospora, Toxocara, and Parvoviral Enteritis groups were lower, the total protein levels of these groups were also lower than the Control and Dietary Diarrhea groups (p<0.0001).
C-reactive protein (CRP) is a potential biomarker of inflammation, infection, and sepsis (
30, 31). CRP levels in dogs are classified as low between 10-50 mg/L, medium between 50-100 mg/L and high if >100 mg/L (
32). In a study conducted on 237 dogs with acute hemorrhagic diarrhea, CRP level was reported as >100 mg/L in 7% of dogs and <50 mg/L in 22% of dogs (
30). The serum CRP levels of the Isospora, Toxocara, and Parvoviral Enteritis groups of the present study were higher than those of the Control and Dietary Diarrhea groups. In addition, the highest CRP level was determined to be in the Parvoviral Enteritis Group among all the other groups (p<0.0001). These findings may be related to the severity of the inflammatory damage in the intestines and the presence of the severe inflammatory response (
2, 3, 30).
This study has some limitations. The lack of histological examination of the intestine and the fact that not all etiologies of AD were covered, such as bacterial-associated diarrhea, may be considered as limitations.
CONCLUSION
Diagnostic tests in dogs with AD are preferred according to the anamnesis, severity of clinical symptoms, and the dog’s clinical stability. Also, performing a microscopic fecal examination with an appropriate method is essential. Although CBC findings are generally normal except for marked eosinophilia in cases of parasitic infestations and leukopenia in cases of CPE, some serum biochemistry abnormalities have been reported in AD cases. These diagnostic tests are useful for forming a differential diagnosis list and demonstrating the damage hematologically. Also, these tests can help assess the possible target organ damage that dehydration may cause. As a result, it was observed that certain parameters related to inflammation, hypoperfusion, and organ damage were higher in CPE and lower in cases of diarrhea due to dietary changes. It was concluded that serum biochemistry parameters can provide more clinical information than CBC in terms of diagnostic differentiation in cases of AD due to different etiologies, especially in triage.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The research was supported by the Faculty of Veterinary Medicine, Harran University, and the Faculty of Veterinary Medicine, Selçuk University.
AUTHORS’ CONTRIBUTION
EG was involved in conceptualization, methodology, investigation, writing-original draft, writing-review and editing; YEE conducted the investigation and writingoriginal draft version

 10.2478/macvetrev-2022-0022
10.2478/macvetrev-2022-0022


