Synthetic detergents which have a major role in environmental pollution accumulate over time and reach levels that harm nature. The surfactants which are abundantly used as cleaning components are discharged into the Van Lake with the sewage water. These chemicals accumulating in the lake may reach a level that could affect the only fish species of the lake, the Van fish. This study aimed to determine the antioxidant levels of Van fish hepatocyte cell culture medium treated with sodium lauryl sulphate (SLS) and to assess the DNA damage. The effect of SLS was assessed by its dose (1x10-5, 1x10-6, 1x10-7 M) and treatment time (24 h, 48 h). Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and DNA damage (8-OHdG) were determined in the SLS hepatocyte culture. SOD and GSH-Px were higher on 24 h and 48 h compared to the control group. A significant increase was observed in CAT level in the first 24 h, especially in 1x10-6 and 1x10-5 M concentration. At 48 h, it was observed that the CAT level decreased significantly as the concentration increased. It was determined that MDA and 8-OHdG levels increased depending on concentration and time. In conclusion, different concentrations of SLS affected antioxidant levels in the primary hepatocyte culture of Van Fish and were found to cause an increase in the levels of MDA and 8-OHdG.
Environmental pollution is a common problem in all nations today. The high accumulation of solid, liquid, and gaseous pollution from various sources in the air, water, and soil is the main reason for environmental pollution. While the advancement of technology provides society with more convenient living conditions, it adds new pollutants to the environment and gradually alters the natural balance. This alters the natural balance of water and threatens the health of aquatic organisms and the people who rely on them for nutrition.
The surfactants have a wide range of use, production, and consumption, and as such are considered as environmental contaminants. The water pollution with these compounds causes considerable adverse effects on living organisms disrupting its interactions (
1, 2). It is difficult to predict the extent of environmental pollution that the surfactants might cause in the future considering the growing trend of their use. Non-biodegradable synthetic detergents are prone to accumulate in aqueous environments when mixed with domestic and industrial wastewater which also contains soil, sea, lake, or river water (
1). Among the most widely used Personal care products is SLS, also identified as Sodium dodecyl sulfate (SDS), an anionic surfactant used as an emulsifying cleaning agent in household detergents such as dishwashing soaps, cosmetics, toothpastes, shampoos, shaving foams, hand soap, facial cleanser, body wash, and shaving creams. In the pharmaceutical industry, SLS is used as an agent to improve the absorption of chemicals through the skin, gastrointestinal mucosa, and other mucous membranes. It is also used as flocculant and de-inking agent in firefighting products, detergents and soaps (
3, 4). Biological oxidation and degradation of the “hard” detergents is relatively long. Detergents have numerous negative effects on biological life in water below their toxic dose (
5).
The understanding of metabolic and enzymatic activities in aquatic organisms as a consequence of pollutants exposure is important in understanding its toxicological effects. Enzymes as physiological and biochemical markers, are used to determine environmental pollution in aquatic organisms (
6). The increase in the concentration of SLS increases the number of free radicals causing oxidative stress in living organisms. Previous studies have demonstrated that SLS exposure in aquatic organisms is manifested by oxidative stress, apoptosis, DNA damage, neurophysiological (
7) and hematological effects, affected swimming performance, and changes in body morphology. In a limited number of studies, detergents were reported to have toxic and allergic effects, and cause the development of cancer cells over time (
1, 8, 9).
There are many protective antioxidant mechanisms that counteract the oxidant degradation caused by free oxygen radicals. The decrease in antioxidant protection causes an increase in reactive oxygen species (ROS), accumulation of free radicals, and consequently adverse changes in the organism. The free radicals trigger oxidation of polyunsaturated fatty acids, proteins, and DNA. This process is defined as oxidative stress and damages normal functions in the body (
10). ROS can affect cellular defense and cause DNA chain fractures, destroying many biochemical mechanisms (membrane ion transport system, enzymes, proteins, lipids). This damage can manifest itself in the form of serious damage to the tissues due to the disturbance in the prooxidant/ antioxidant balance. Numerous damage-products caused by DNA oxidation have been identified. The guanine derivative 8-OHGua and its deoxynucleoside 8-OHdG are more important in the detection of DNA damage (
11).
Van fish is in the second place after carp in inland fish production in Turkey. It is an important source of protein for the people of the region. In addition, Van fish is an endemic fish species that can live at pH 9.8. Due to these features, it has a great economic importance. Therefore, the aim was to investigate the effect of SLS on the antioxidant enzymes (SOD, GSH-Px and CAT), lipid peroxidation (malondialdehyde, MDA), and DNA damage (8-OHdG level) in liver cultures of Van fish.
MATERIAL AND METHODS
FishThe Van fish used in the study were caught with nets from the Lake Van. The fish brought to the laboratory were studied after a 10-day adaptation period. A total of 10 fish were sampled, 5 females and 5 males. The study was approved by the Van Yüzüncü Yıl University, Experimental Animals Local Ethics Committee (Decision number 2017/05).
Cell cultureThe liver tissue was removed after dissection in laminar flow cabinet. The liver tissue was treated with hepatocyte buffer (Hepatocyte Buffer: 0.145 M NaCl, 5.4 mM KCl, 5 mM EDTA, 1.1 mM KH
2PO
4, 12 mM NaH
2CO
3, 3 mM NaHPO
4, pH=7.6.). After repeating this process several times over a 30-minute period, the tissue was placed in an EDTA-free hepatocyte buffer containing 2.5 mM CaCl
2. Thus, the blood-cleaned tissue was incubated for 30 minutes in collagenase containing L-15 (0.5 mg/mL). After incubation, the tissue was triturated twice and centrifuged at 90 x g for 5 minutes until the resulting supernatant became clear. Antibiotic-antimycotic solution (10 mL/L) and 5 mM NaHCO
3 were added at the end of the centrifugation by removing the supernatant and replacing the feed medium (Leibovitz-15) to prevent contamination. The isolated cells were stained with trypan blue (4%) to distinguish between live and dead cells (
12). The erythrocyte number in the culture was <10%. The isolated hepatocytes were placed in appropriate cell culture vessels and SLS was applied at concentrations of 1x10
-5, 1x10
-6, and 1x10
-7 M. Tissues were extracted into cell cultures at 24 and 48 hours. The supernatants obtained as a result of the extraction were examined by applying the methods described in the following text.
Analysis of antioxidant enzymesSOD enzyme activity was measured at 505 nm at 37 °C in an autoanalyzer with the Randox -Ransod enzyme kit (13, 14). CAT enzyme activity was determined at 240 nm by the UV spectrophotometric method of Aebi (
15) based on the degradation of hydrogen peroxide (H
2O
2) by catalase. GSH-Px enzyme activity was measured at 37 ºC with Randox- Ransel enzyme kits in an autoanalyzer with the 340 nm ultraviolet method (
16, 17).
Analysis of 8-OHdG levelsHomogenates were prepared according to Alak et al. (
18) with minor modifications. After the necessary solutions were added to the obtained homogenates and washing processes were applied, the remaining kit procedures were performed. After adding the stop solution for reading on the ELISA device, three readings were taken for reliability at 450 nm within 10 minutes which were averaged (ELISA kit, Catalog No: 201-00- 0041/SunRed).
Analysis of Malondialdehyde (MDA) levelsHomogenates for analysis were prepared according to the modified method of Mis et al. (
19). MDA level was measured by the method of Placer et al. (
20) based on the spectrophotometric measurement of pink color absorbance resulting from the thiobarbituric acid (TBA) and MDA reaction.
Statistical analysisThe results are expressed as mean ± standard error. One-way analysis of variance (ANOVA) and Duncan’s multiple comparison tests were applied considering significance level of p<0.05. SPSS 23 software was used for statistical calculations.
RESULTS
The SOD level changed depending on the concentration. It reached a significantly higher level at 1x10
-5 SLS concentration at 48 h (p<0.05) (
Fig. 1).
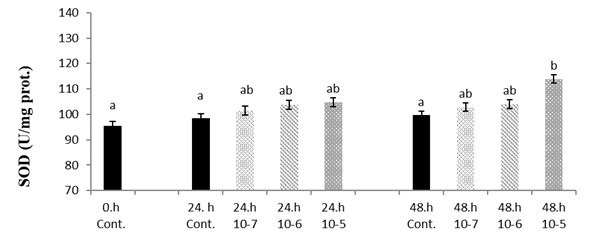 Figure 1.
Figure 1. Van fish hepatocyte culture exposed to SLS, time and dose dependent variation of SOD level. The letters a, b, c, and d indicate a significant difference between the groups (p<0.05)
The GSH-Px level has significantly increased with the time depending on the concentration (p<0.05) (
Fig. 2).
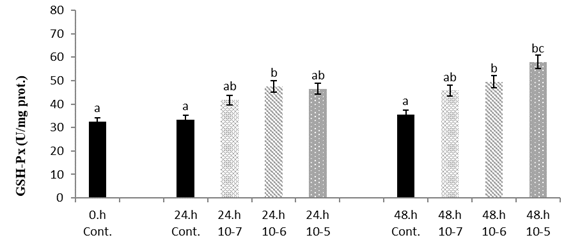 Figure 2.
Figure 2. Van fish hepatocyte culture exposed to SLS, time and dose dependent variation of GSH-Px level. The letters a, b, c, and d indicate a significant difference between the groups (p<0.05)
It was observed that CAT level was significantly higher on 1x10
-6 a nd 1x10
-5 SLS concentration at 24 h compared to the control. Significantly lower CAT levels were observed at 48 h in all three groups (p<0.05) (
Fig. 3).
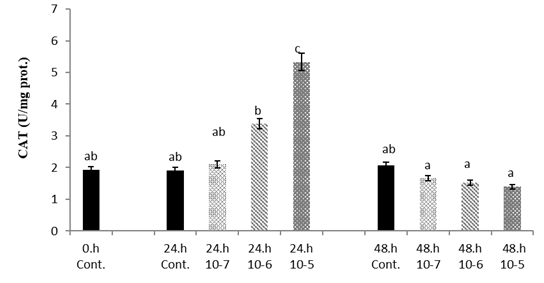 Figure 3.
Figure 3. Van fish hepatocyte culture exposed to SLS, time and dose dependent variation of CAT level. The letters a, b, c, and d indicate a significant difference between the groups (p<0.05)
MDA showed significantly higher level with increasing SLS concentrations at 24 h and 48 h (p<0.05) (
Fig. 4).
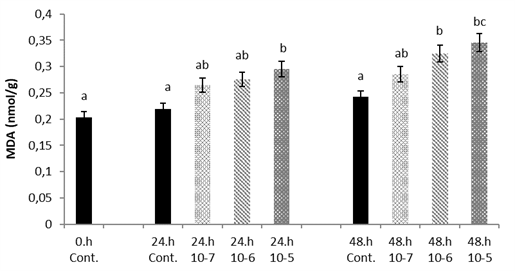 Figure 4.
Figure 4. Van fish hepatocyte culture exposed to SLS, time and dose dependent variation of MDA level. The letters a, b, c, and d indicate a significant difference between the groups (p<0.05)
There was significantly higher 8-OHdG level in the hepatocyte cell culture treated with 1x10
-5 and 1x10
-6 SLS concentrations, especially at 48 h (p<0.05) (
Fig. 5).
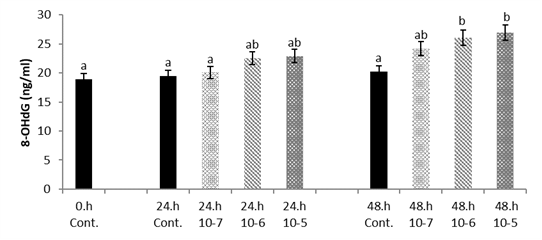 Figure 5.
Figure 5. Hepatocyte cultures exposed to SLS, time and dose change of Van fish hepatocyte culture exposed to SLS, time and dose dependent variation of 8-OHdG level. The letters a, b, c, and d indicate a significant difference between the groups (p<0.05)
DISCUSSION
The SLS application to Van fish liver culture medium was observed to increase lipid peroxidation and DNA damage (8-OHdG) depending on its concentration. The antioxidant levels counteracted the free radicals formed by the effect of SLS but this was varying depending on the SLS concentration. The CAT enzyme primarily functions in peroxisomes which are located in a large number of liver tissues. The time-dependent decrease in CAT level in the hepatocyte culture may be due to denaturation or secretion inhibition caused by the SLS toxicity.
Sayed and Authman (
21) aimed to assess the effects of SLS and the potential influence of
Spirulina platensis in African catfish. They reported hepatic and kidney dysfunction, electrolyte imbalance, significant deterioration in enzymatic and non-enzymatic antioxidants, and an increase in apoptosis percentages. In another study including herb carp, it was observed that there was high microbial activity, high level of gill tissue damage and an increase in antioxidant levels (SOD and CAT) (
22). Yakovenko et al. (
23) studied the effect of SLS (pure and as part of detergents) on enzymes activity in energy pathways in carp reporting significantly negative changes. The current study demonstrated increased antioxidant activity in Van fish exposed to SLS which is in compliance with the previous reports.
It was determined that the levels of SOD and CAT in the liver and muscle tissues of Nile tilapia fish exposed to different doses of SLS (0.0625, 0.125, 0.25, 0.5, 1 g/L) began to decrease (
24). Jifa et al. (
25) applied SLS and sodium dodecyl benzene sulfonate to Japanese perch and have reported that fish attempt to tolerate oxidative stress with different levels of SOD, CAT, GSH-Px in the liver tissue. SLS applied to the
Mytilus galloprovincialis triggered oxidative stress, increased ROS, and significantly increased SOD and CAT activity (
26). Similarly, Suganthi et al. (
27) reported high antioxidant activity in
C. quinquecirrha strain following treatment with SLS. The current study demonstrated that Van fish liver culture cells respond by increased metabolic rate and antioxidant activity following SLS treatment. These findings are in compliance with numerous other reports.
Jung et al. (
28) reported a significant amount of SLS absorption in intestinal tissues.
Pseudosida ramosa species lost some of its functions after 21 days treatment with SLS depending on its concentration (
29). Mei et al. (
30) reported increased lipid peroxidation (MDA) in a mixture containing salmon oil by applying anionic SLS. Lipids are the first reserve to be used by organisms when submitted to stressful conditions. Cell membrane lipids under such conditions can be easily peroxidized by free radicals (
31). Alak et al. (
32) reported that apoptosis and DNA damage formation are important markers of oxidative stress resulting from toxicity (
33, 34). The current study has also demonstrated DNA damage as a consequence of SLS toxicity. The toxic effects of SLS in aquatic organisms are very limited, thus the full extent of negative effects should be further elucidated.
CONCLUSION
The antioxidant level (SOD, CAT, GSH-Px), MDA, and DNA damage (8-OHdG) were negatively affected by SLS application in Van fish liver culture cells. The SLS concentration and time of exposure may be considered as significant factors of its toxic effects. SLS is a prooxidant compound. Further studies are required to elucidate the full extent of SLS toxicity in fish. The toxic effects of SLS on living organisms may cause more complex results in the future due to changes in environmental conditions and increasing amount of SLS. Therefore, future studies should address more realistic exposure scenarios for a more relevant risk assessment.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
This study was supported by the project of Van Yuzuncu Yil University, Scientific Research Projects with FBA-2018-6378 project.
AUTHORS’ CONTRIBUTION
ACY did the experiment and wrote the article. ARO did the cell culture of the study.

 10.2478/macvetrev-2022-0027
10.2478/macvetrev-2022-0027




