Abstract
The incidence of clinical mastitis (CM) in small and large-scale dairy cow farms can be highly variable and can be affected by age, parity, post-calving status, and atmospheric conditions. The current study aimed to assess the CM-incidence and its association with the post-calving days, number of lactations, season, and number of affected udder quarters in dairy cows in small-scale dairy farms. The study was conducted within one calendar year in 177 small-scale farms with 864 dairy cows. Clinically confirmed CM cows (n=72) were sampled from each udder quarter and processed for bacteriology examination. The CM-positive samples were grouped according to the season (spring, summer, autumn, and winter), the number of days after calving (<90, 90-180, and >180), the number of lactations (1-st, 2-5-th, and >6-th), and the number of affected udder quarters (one, two, three, and four). The CM-positive samples (n=72, 8.3%) were confirmed on bacteriology examination in significantly lower count (n=56, 6.5%). The 2-5-th lactations cows (n=35, 68.6%) were significantly more compared to the first lactation (n=6, 11.8%), and >6-th lactation cows (n=10, 19.6%). CM cows with infection of one udder quarter (n=40, 78.4%) were significantly more than the cows with two (n=6, 11.8%), and four infected quarters (n=5, 9.8%). The CM-incidence in small-scale dairy cow farms in North Macedonia was 8.3% and 6.5% by clinical and bacteriology examination, respectively. The highest CM susceptibility was observed in the cows between the second and fifth lactations. One udder quarter was most frequently affected in CM-positive cases.
Keywords: mastitis, cows, lactation, calving, parity
INTRODUCTION
Milk and dairy products are globally considered a basic food source. In order to meet the high demand for dairy products, the farmers aimed to increase the daily yield of milk in the cows and to increase to the herd size (
1). The physiological consequence on the udder health was reflected by increased susceptibility to mastitis and other udder-related health issues (
2).
The mastitis is considered as frequent and economically highly expensive disease especially affecting the moderate and high-yield dairy cows in the peripartum period (
3, 4, 5). The clinical manifestation of mastitis (latent, subclinical, or clinical) is highly correlated with the speed of the immune response reflected by the polymorphonuclear leucocyte migration in the affected udder quarter (
6, 7). The clinical mastitis (CM) is defined as udder alveolar parenchyma inflammation manifested by pain, edema, fibrosis, and udder induration, which is reflected by altered physical, chemical, and microbiological composition of the milk (
5, 8). This condition affects the economic revenue, renders the milk unfit for consumption and processing, and affects animal health welfare, and longevity (
8, 9).
The highly variable reports on the CM incidence are due to different methods for identifying and categorizing mastitis cases and due to the various levels on which the studies were conducted (e.g., geographical area, number of dairy farms, time interval of sampling, etc.) (
10, 11). The yearly incidence of CM in the EU countries was reported from 21% to 50% (
12, 13). In North Macedonia, the latest report declares 34% CM-positive cases, but the findings must be regarded with reserve since it was conducted on 3 farms (
10). However, these data illustrate the CM incidence in large-scale dairy cattle farms dispersed in larger geographical area. We hypothesized that the CM incidence in lowscale farms located on small geographical and same epizootic area will be lower than 20%.
CM incidence was positively correlated with the parity being reported as 15% in the first, 18% in the second, and 22% in the third lactation (
16). The early post-calving period, especially in the first 14 days, was reported to be most critical (
10, 17, 18) regardless of the age or parity. The high atmospheric temperature in the summer, the high humidity in spring and autumn, and unventilated stable environment in winter are correlated with the CM occurrence (
10, 14, 19, 20). It was hypothesized that higher CM occurrence will be observed in early days following parturition, in cows between the first and fifth lactations, and in the spring, summer, and winter season. Also, it was hypothesized that one udder quarter will be most frequently affected in CM-positive dairy cows.
The study aimed to assess the CM-incidence and its association with the post-calving days, number of lactations, season, and number of affected udder quarters in dairy cows in small-scale dairy farms.
MATERIAL AND METHODSТhe research was conducted throughout the whole calendar year of 2020. Dairy cows (N=864) from 177 small-scale dairy farms were included in the study housing from 1 to 36 animals in each. They were located in a small geographical area (28 km range), belonging to the same epizootic region, and serviced by a single veterinary clinic. All cows were considered to be samples from a single pool since the type of feeds, micromanagement and housing (tie stall barns, automated milking, etc.), microclimatic conditions, epizootic environment, and veterinary care were similar.
Cows which were suspected for mastitis by the owners were subjected to inspection by the veterinarian employing clinical examination, and if positive, additional bacteriology examination of milk samples was conducted. The cows which showed udder redness and edema, watery milk, occurrence of small or large milk coagulation particles, presence of blood or pus in the milk, and altered overall health status were considered to be clinically positive on mastitis (CM). The CM-positive cows were then milksampled from each udder quarter for bacteriological examination according to the National mastitis council protocol 2004 (
21). The samples were packed in sterile tubes (15 mL), marked and frozen to -80 °C until laboratory analysis.
The bacteriology analysis of the collected samples was conducted by applying 10 uL of sample on blood agar with 5% sheep blood (blood agar base, Biolife, USA). The plates were incubated on 37 °C in aerobic conditions for 24 and 48 hours. The bacteria colony count was performed microscopically without its identification. Uniform growth of colonies and CFU>10 was considered as positive bacteriology finding of the samples.
The CM-positive cows were grouped according to the season (spring, March-May, summer, June-August, autumn, September-November, and winter, December-February) (
10), the number of days after calving (<90, 90-180 days, and >180 days), the number of lactations (1, 2-5, and ≥6 lactations), and the number of affected udder quarters (one, two, three, and four udder quarters).
The
a priori power analysis was performed by considering value of power ≥0.95 for the respective degrees of freedom (df) and the number of sample counts (G*Power v.3.9.1.2., Franz Faul, Kiel University, Germany). The counts in each respective groups were compared by chi-square test of independence (df ≥2). The binomial distribution of positive and negative cases in the clinical and bacteriology examination was conducted by employing Mc Nemar’s chi square test (df=1) (IBM, SPSS, USA). The level of significance was p<0.05.
RESULTS
The
post hoc power analysis for the chi-square test of independence for the comparisons with df >1 and N=200 was >0.95. For the Mc Nemar’s chi-square test post hoc test with df=1, N=864 the
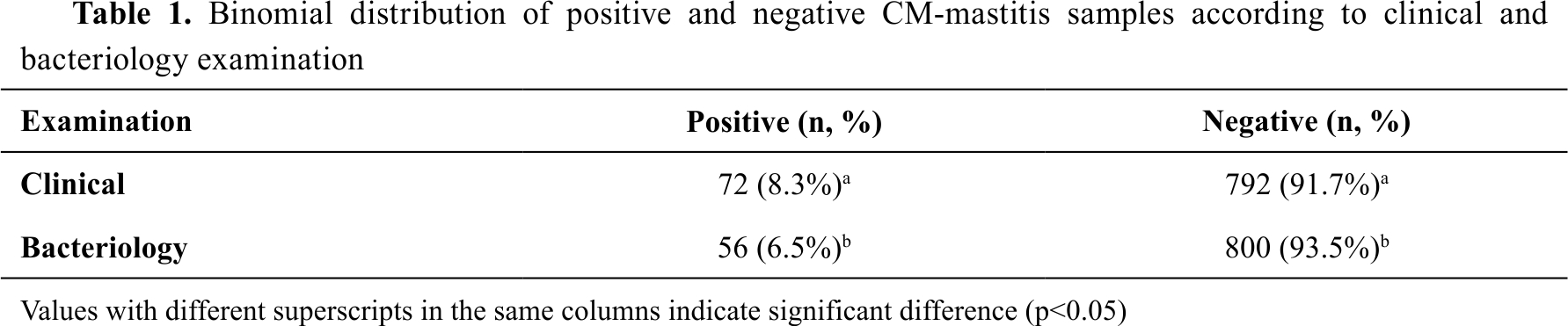
post hoc power was estimated >0.99. In the CM prevalence analysis (N=864), the clinical examination positive counts (n=72, 8.3%) was by 1.8% significantly higher compared to the bacteriology positive counts (n=56, 6.5%) (
Table 1).
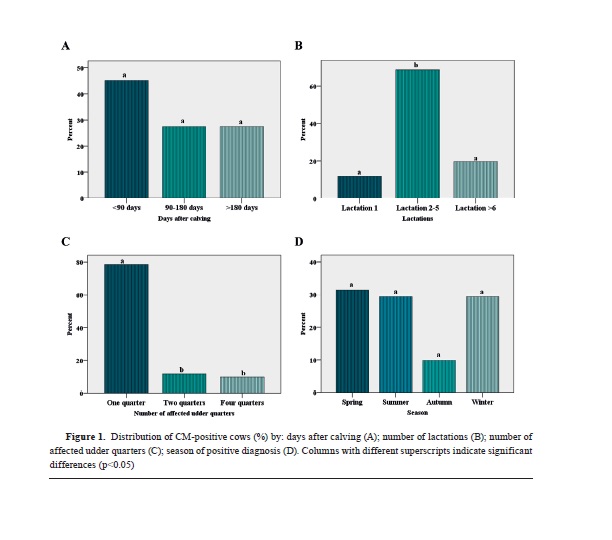
In the analysis for the incidence of CM in dairy cows on the days following calving (N=200), there was non-significantly higher count of cows in the <90 days group (n=23, 45.1%) compared to the 90-180 days (n=14, 27.5%), and >180 days (n=14, 27.5%) (
Fig. 1A).
In the analysis for the number of lactations on the incidence of CM (N=200), there was significantly higher count in the 2-5 lactations (n=35, 68.6%) compared to the 1 lactation (n=6, 11.8%) and >6 lactations (n=10, 19.6%) group (
Fig. 1B).
In the analysis for the number of affected udder quarters for the CM-positive cases (N=200), it was observed that the one quarter group (n=40, 78.4%) had significantly higher count compared to the two (n=6, 11.8%) and four quarter group (n=5, 9.8%) (
Fig. 1C).
In the analysis for the season and occurrence of CM in dairy cows (N=200), it was observed that in autumn (n=5, 9.8%) there was non-significantly lower count compared to the spring (n=16, 31.4%), summer (n=15, 29.4%), and winter (n=15, 29.4%) season group (
Fig. 1D).
DISCUSSION
The CM incidence in the current study was 8.3% on a yearly basis. This finding was lower compared to studies conveyed in Uruguay 14.4% (
22), China 37.0% (
20), Denmark 42.0% (
23), and North Macedonia 34.0% (
10). The reason behind this high difference between the current and other reports could be explained due to the fact that a very high number of small-scale dairy farms were included (N=177) which had in average 5 cows. In this type of farms, the owners are in everyday contact with the cows during the milking process and are able to notice udder and milk changes in the early phases of mastitis occurrence. Since they don’t have high workload, they are able to apply early treatment (e.g., frequent milking, cold compress, ointments, etc.) which halts or eases the mastitis progression. If the symptoms are relieved, the farmers are not requesting professional veterinary care thus the cows are not being reported as affected by mastitis to the veterinarian. Additionally, the higher somatic cells count in these cows are reported to be positively correlated with CM resistance (
7, 24, 25). In our preliminary unpublished trial, it was observed that the average somatic cell count in small-scale dairy farms was in the range between 8 and 8,908х10
3 cells/mL.
The CM incidence in this study was nonsignificantly lower in autumn compared to the other seasons. Other studies reported that the high CM incidence was in spring and autumn (
10), from March to October (
19), or in April, May, July, August, and December (
20). These reports are concluding that the high atmospheric temperature in summer and the high humidity in spring and autumn are correlated with the CM occurrence. Additionally, these conditions are favoring mastitis-pathogens replication and spreading through the wet floor and bedding (
26, 27). The relatively higher CM-positive counts in summer could be explained due to the high atmospheric temperatures, and in winter due to the high environmental temperatures, and the lack of sufficient sunlight and appropriate ventilation in the farms. In early spring, the green feed causes watery excrements which lowers the barns hygienic conditions and increases pathogens retention. The relatively lower CM cases in autumn could be explained due to the relatively lower and optimal environmental temperatures, and the introduction of winter-feed (balanced alfalfa hay) renders the excrement in a more solid state increasing the hygienic conditions.
CM incidence was positively correlated with the parity and hence the number of lactations (
15, 18, 26). CM incidence was reported as 15% in the first, 18% in the second, and 22% in the third lactation (
16). In another report, the CM incidence in the first lactation was 11%, progressively increasing to 19% in the third lactation, and then starting do decrease with the consequent lactations (
2). The highest CM incidence was reported in dairy cows at age range of 3.5-4.5 years (
28).
Similarly, the current study observed that the cows in the 2-5 lactation cycle had highest CM incidence compared to the 1-lactation and >6-lactation cows, whereas the >6 lactation cows were with non-significantly higher count compared to the 1-lactation cows. However, the first lactation cows tend to have higher susceptibility at the beginning of the lactation (
11). The increasing age of the dairy cows affects the udder susceptibility to mastitis infection due to the anatomical alterations, lowering and widening of the udder teat openings (
9), frequent contact of the teats with the floor, and frequent physical injuries (
9, 29). Also, the increasing age lowers the physiological leucocyte response (
30).
CM incidence is high in the early post-calving period (
10, 18). About 43% of CM cases occur in the early lactation (
31) or in the first 14 days of the lactation (
17). In the current study, it was observed that there were non-significantly higher CM cases in the period between 0-3 months by 17.6% compared to the 3-6 and >6 months. Cows in the first calving period have lower CM incidence, but with some predisposition towards the beginning of the lactation due to the udder edema (
1, 11). Additionally, the placenta retention, pyometra, endometritis, twinning, and metabolic disorders could contribute to the higher CM predisposition in first-calving cows (
32, 33). The contributing pathophysiological mechanisms include hypocalcemia which decreases the teat sphincter tonus, the stress and hypercortisolemia cause immunosuppression effect, and the negative energy balance decreases the phagocytic function of the polymorphonuclear leucocytes and affects the lymphocyte function (
34).
One udder quarter was most frequently affected in CM-positive cows. Other reports have similar findings with 74% (
2) or 69 and 75% (
19) of the positive samples with one udder quarter affected. The subclinical mastitis however, affects more than one udder quarter. In one study, it was found that positive cows on subclinical mastitis had one, two, three, and four affected udder quarters in 60%, 29%, 3.2%, and 8%, respectively (
10). Another study reported 47%, 34%, 12%, and 6.4%, respectively (
26). The highly contagious causative agents of the subclinical mastitis (
Staphylococcus aureus and
Streptococcus agalactiae) are the reason behind the major differences in the number of affected udder quarters with the CM. The CMpositive cows in this study were in the period <90 days after calving which indicates that they might have had been infected before the drying off.
The CM samples (n=72, 8.3%) were confirmed on bacteriology examination in significantly lower count (n=56, 6.5%). Lower bacteriology findings compared to the CM samples were also reported in Canada by 18% (
18), and in Sweden and the Flaman region by 20% (
11, 34). Conversely, higher bacteriology findings compared to the CM samples were reported in Uruguay and Italy (Ragusa) by 33% and 40%, respectively (
22, 35).
Тhese variations could be explained due to several reasons: medication retention in the milk, presence of highly resistant bacteria, antibiotic bacterial inhibition, reducing of bacterial count between the period of sampling and analysis, specific bacterial cultivation, short-term infections (
23, 28, 32, 34, 36). The minimum CFU/mL in the milk should be >100 for successful bacterial isolation (
35). The lower CFU/mL count, low infection rate, or high phagocytic activity of the polymorphonuclear leucocytes might render the sample more difficult to isolate the bacteria. Additionally, the variations in the sampling, transport, time, and storage conditions could contribute to the differences of the bacterial isolation rate. The freezing might be beneficial for some (
Staphylococcus spp. and
Streptoccocus spp.) and deleterious for other bacteria (
Esherichia coli) (
18). This can affect the isolation success of various causative agents of CM in affected farms.
Despite the variable CM prevalence reports in dairy cow farms, the following preventive measures should be undertaken in order to minimize the risk of its occurrence: regular farmer training and education, hygiene monitoring before, during, and after milking, udder health monitoring, and control of milk hygiene and cold chain (
37).
CONCLUSION
The CM incidence in small-scale dairy farms was 8.3% in North Macedonia. The bacterial isolation was successful in 77.77% of the samples obtained from CM-positive cases. There was higher but non-significant tendency for CM occurrence in the first 90 days following calving. The highest CM susceptibility was observed in the cows between the second and fifth lactations. One udder quarter was most frequently affected in CM-positive cases. There was lower tendency for CM in the autumn season but it was non-significant.
CONFLICT OF INTEREST
The authors declared that they have no potential conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Faculty of Veterinary Medicine–Skopje, Ss. Cyril and Methodius University in Skopje, N. Macedonia. The authors express their gratitude to Veterinary Clinic “Pro-Infovet” Petrovec, Skopje, for collecting the samples.
AUTHORS’ CONTRIBUTION
AJ developed the study design and concept, collected the samples, and was involved in manuscript writing. MN was involved in defining the hypothesis, statistical analysis, and manuscript writing. SA was responsible for sample and dairy cow data management. AT performed sample collection. IC was included in bacteriology analysis and interpretation. DM supervised the study, hypothesis testing and results interpretation.
References
1. De Vliegher, S., Fox, L.K., Piepers, S., McDougall, S., Barkema, H.W. (2012). Invited review: Mastitis in dairy heifers; nature of the disease, potential impact, prevention, and control. J Dairy Sci. 95(3): 1025-1040.
https://doi.org/10.3168/jds.2010-40742. Nakov, D., Trajcev, M. (2012). Udder quarter risk factors associated with prevalence of bovine clinical mastitis. Mac Vet Rev. 35(2): 55-64.
3. Tomanić, D., Božin, B., Čabarkapa, I., Kladar, N., Radinović, M., Maletić, M., Kovačević, Z. (2022). Chemical composition, antioxidant and antibacterial activity of two different essential oils against mastitis associated pathogens. Acta Vet. 72(1): 45-58.
https://doi.org/10.2478/acve-2022-00044. Taponen, S., Liski, E., Heikkilä, A.M., Pyörälä, S. (2017). Factors associated with intramammary infection in dairy cows caused by coagulasenegative staphylococci, staphylococcus aureus, streptococcus uberis, streptococcus dysgalactiae, corynobacterium bovis, or escherichia coli. J Dairy Sci. 100(1): 493-503.
https://doi.org/10.3168/jds.2016-11465 PMid:28341052
5. Shaheen, M., Tantary, H.A., Nabi, S.U. (2016). A treatise on bovine mastitis: disease and disease economics, etiological basis, risk factors, impact on human health, therapeutic management, prevention and control strategy. J Adv Dairy Res. 4, 1-10.
6. Neijenhuis, F., Barkema, H.W., Hogeveen, H., Noordhuizen, J.P.T.M. (2001). Relationship between teat-end callosity and occurrence of clinical mastitis. J Dairy Sci. 84(12): 2664-2672.
https://doi.org/10.3168/jds.S0022-0302(01)74720-0 PMid:11814022
8. Hristov, S., Stanković, B., Relić, R. (2005). Clinical and subclinical mastitis in cows. Biotechnol Anim Husb. 21(1-2): 29-39.
https://doi.org/10.2298/BAH0502029H 9. Guarín, J.F., Paixão, M.G., Ruegg, P.L. (2017). Association of anatomical characterisics of teats with quarter-level somatic cell count. J Dairy Sci. 100(1): 643-652.
https://doi.org/10.3168/jds.2016-11459 PMid:27816244
10. Trajcev, M., Nakov, D., Hristov, S., Andonov, S., Joksimovic-Todorovic, M. (2013). Clinical mastitis in macedonian dairy herds. Acta Vet. 63(1): 63-76.
https://doi.org/10.2298/AVB1301063T 11. Verbeke, J., Piepers, S., Supré, K., De Vliegher, S. (2014). Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J Dairy Sci. 97(11): 6926-6934.
https://doi.org/10.3168/jds.2014-8173 PMid:25218745
12. Bartletta, P.C., Agger, J.F., Houe, H., Lawson, L.G. (2001). Incidence of clinical mastitis in Danish dairy cattle and screening for non-reporting in a passively collected national surveillance system. Prev Vet Med. 48(2): 73-83.
https://doi.org/10.1016/S0167-5877(00)00192-6 PMid:11154781
13. Plym-Forshell, K., Osteras, O., Aagaard, K., Kulkas, L. (1995). Disease recording and cell count data in 1993, in Sweden, Norway, Denmark and Finland. Proceedings of the 3rd International Mastitis Seminar, Session 4, 50-54. May, Tel Aviv, Israel
14. Trajcev, M. (1996). Research of globulins in the colostrum and immunoglobulins in the blood serum of calves depending on the hygiene of cows. pp. 78. Faculty of Agriculture, Ss. Cyril and Methodius University - Skopje. Master thesis. [In Macedonian].
15. Nyman, A.K., Persson Waller, K., Bennedsgaard, T.W., Larsen, T., Emanuelson, U. (2014). Associations of udder-health indicators with cow factors and with intramammary infection in dairy cows. J Dairy Sci. 97(9): 5459-5473.
https://doi.org/10.3168/jds.2013-7885 PMid:24997662
16. Carlen, E., Emanuelson, U., Strandberg, E. (2006). Genetic evaluation of mastitis in dairy cattle using linear models, threshold models, and survival analysis: A simulation study. J Dairy Sci. 89(10): 4049-4057.
https://doi.org/10.3168/jds.S0022-0302(06)72448-1 PMid:16960081
17. Barkema, H.W., Schukken, Y.H., Lam, J.T., Beiboer, L.M., Benedictus, G., Brand, A. (1998). Management practices associated with low, medium, and high somatic cell counts in bulk milk. J Dairy Sci. 81(7): 1917-1927.
https://doi.org/10.3168/jds.S0022-0302(98)75764-9 PMid:9710760
18. Sargeant, J.M., Scott, H.M., Leslie, K.E., Ireland, M.J., Bashiri, A. (1998). Clinical mastitis in dairy cattle in Ontario: frequency of occurrence and bacteriological isolates. Can Vet J. 39(1): 33-38. 19. Stojanovski, S., Cilev, G., Trajanovska, B. (2021). Bacteria variety causing clinical mastitis in Holstein-Friesian cows in Pelagonia region, North Macedonia. Agric Sci Technol. 13(3): 307-312.
https://doi.org/10.15547/ast.2021.03.051 20. Gao, J., Barkema, H.W., Zhang, L., Liu, G., Deng, Z., Cai, L., Shan, R., et al. (2017). Incidence of clinical mastitis and distribution of pathogens on large Chinese farms. J Dairy Sci. 100(6): 4797-4806.
https://doi.org/10.3168/jds.2016-12334 PMid:28434736
22. Gianneechini, R., Concha, C., Rivero, R., Delucci, I., López, M.J. (2002). Occurrence of clinical and subclinical mastitis in dairy herds in the West Littoral Region in Uruguay. Acta Vet Scand. 43(4): 221-230.
https://doi.org/10.1186/1751-0147-43-221 PMid:12831175 PMCid:PMC1764198
23. Bartlett, P.C., Agger, J.F., Houe, H., Lawson, L.G. (2001). Incidence of clinical mastitis in Danish dairy cattle and screening for non-reporting in a passively collected national surveillance system. Prev Vet Med. 48(2): 73-83.
https://doi.org/10.1016/S0167-5877(00)00192-6 PMid:11154781
24. Dosogne, H., Capuco, V.A., Paape, J.M., Roets, E., Burchevich, C., Fenwick, B. (1988). Reduction of acyloxyacyl hydrolase activity in circulating neutrophils from cows after parturition. J Dairy Sci. 81(3): 672-677.
https://doi.org/10.3168/jds.S0022-0302(98)75622-X PMid:9565869
25. Beaudeau, F., Fourichon, C., Seegers, H., Bareille, N. (2002). Risk of clinical mastitis in dairy herds with a high proportion of low individual milk somatic-cell counts. Prev Vet Med. 53(1-2): 43-54.
https://doi.org/10.1016/S0167-5877(01)00275-6 PMid:11821136
27. O’Driscol, K., Boyle, L., Meaney, B., Hanlon, A. (2008). The effect of out-wintering pad design on dirtiness score, somatic cell score and mastitis incidence in dairy cows. Animal 2(6): 912-920.
https://doi.org/10.1017/S1751731108001882 PMid:22443671
28. Khokon, M.S.I., Azizunnesa, M., Islam, M.M., Chowdhury, K.B., Rahman, M.L., Ali, M.Z. (2017). Effect of mastitis on postpartum conception of cross bred dairy cows in Chittagong district of Bangladesh. J Adv Vet Anim Res. 4(2): 155-160.
https://doi.org/10.5455/javar.2017.d203 29. Breen, J.E., Green, M.J., Bradley, A.J. (2009). Quarter and cow risk factors associated with the occurrence of clinical mastitis in dairy cows in the United Kingdom. J Dairy Sci. 92(6): 2551-2561.
https://doi.org/10.3168/jds.2008-1369 PMid:19447987 PMCid:PMC2690977
32. Svensson, C., Nyman, A.K., Waller, K.P., Emanuelson, U. (2006). Effects of housing, management, and health of dairy heifers on firstlactation udder health in Southwest. Sweden. J Dairy Sci. 89(6): 1990-1999.
https://doi.org/10.3168/jds.S0022-0302(06)72266-4 PMid:16702262
33. Kirovski, D., Šamanc, H., Vujanac, I., Prodanović, R., Sladojević, Ž. (2010). Metabolic diseases and udder health. Proceedings of the 12 Regional meeting in clinical pathology and animal therapy “Clinica veterinaria 2010”. June, 18-20 (pp. 14-17), Subotica, R. Serbia [In Serbian]
34. Persson, Y., Nyman, A.K.J., Grönlund-Andersson, U. (2011). Etiology and antimicrobial susceptibility of udder pathogens from cases of subclinical mastitis in dairy cows in Sweden. Acta Vet Scand. 53(1):36.
https://doi.org/10.1186/1751-0147-53-36 PMid:21649936 PMCid:PMC3118135
35. Ferguson, J.D., Azzaro, G., Gambina, M., Licitra, G. (2007). Prevalence of mastitis in Ragusa, Sicily, from 2000 to 2006. J Dairy Sci. 90(12): 5798-5813.
https://doi.org/10.3168/jds.2006-903 PMid:18024774
36. Abdel-Rady, A., Sayed, M. (2009). Epidemiological studies on subclinical mastitis in dairy cows in Assiut Governorate. Vet World. 2(10): 373-380.
https://doi.org/10.5455/vetworld.2009.373-380 37. Mihajlović, L., Cincović, M., Nakov, D., Stanković, B., Miočinović, J., Hristov, S. (2022). Improvement of hygiene practices and milk hygiene due to systematic implementation of preventive and corrective measures. Acta Vet. 72(1): 76-86.
https://doi.org/10.2478/acve-2022-0006

 10.2478/macvetrev-2023-0010
10.2478/macvetrev-2023-0010

