Swab samples were collected from 100 dogs of different age, sex and breed diagnosed with otitis externa, admitted to the Istanbul University- Cerrahpaşa, Faculty of Veterinary Medicine clinics between October 2019 and March 2020. The samples were placed in Stuart’s transport medium and brought to the laboratory in a cold chain. (Istanbul University-Cerrahpaşa Rectorate Veterinary Faculty Unit Ethics Committee Presidency, Report no: 2019/10).
The swabs were inoculated in Mannitol Salt Agar and incubated at 37 °C for 24 hours. Suspected
spp. isolates were initially isolated and identified using standard biochemical tests (
). BD Phoenix Automated Microbiology System (BD Diagnostic Systems, Sparks, MD) was used for bacterial identification and antimicrobial susceptibility testing according to the manufacturer’s instructions. Isolates with resistance to three or more than three of the fluoroquinolone, aminoglycoside, lincosamide, sulphonamide, tetracycline, macrolide, and β-lactam antibiotic groups were classified as having multiple drug resistance (MDR).
All statistical analyses were conducted using SPSS Statistics version 25 (IBM, USA). Statistical significance was set at p<0.05.
spp. was isolated as pure culture in 36% of the samples.
spp. was detected in 15 (15/52, 28.8%) male and 21 (21/48, 43.75%) female dogs.
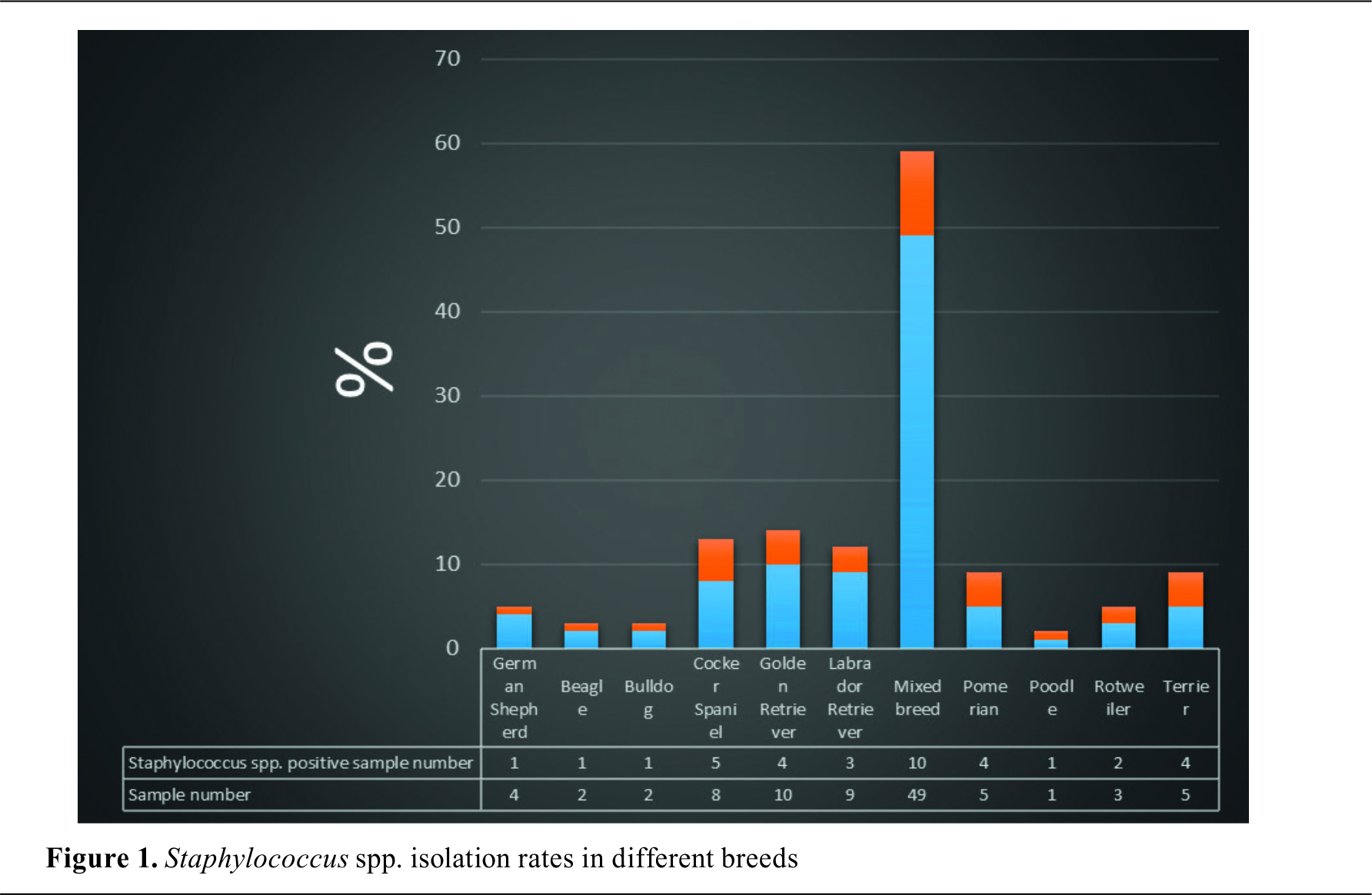
spp. positive samples were distributed in the following age groups: <1 year (9/26, 34.6%), 1-8 years (22/63, 34.9%), >8 years old (5/11, 45.4%). The presence of OE was found to be 80.0% in Terriers, 80.0% in Pomeranians, 66.6% in Rottweilers, 62.5% in Cocker Spaniels, 33.3% in Labradors, 25.0% in Golden Retrievers and 20.4% in mixed breeds. The distribution of
spp. positive samples according to the breed is shown in
. The isolates’ distribution according to the
. No significant differences were observed (p>0.05).
.
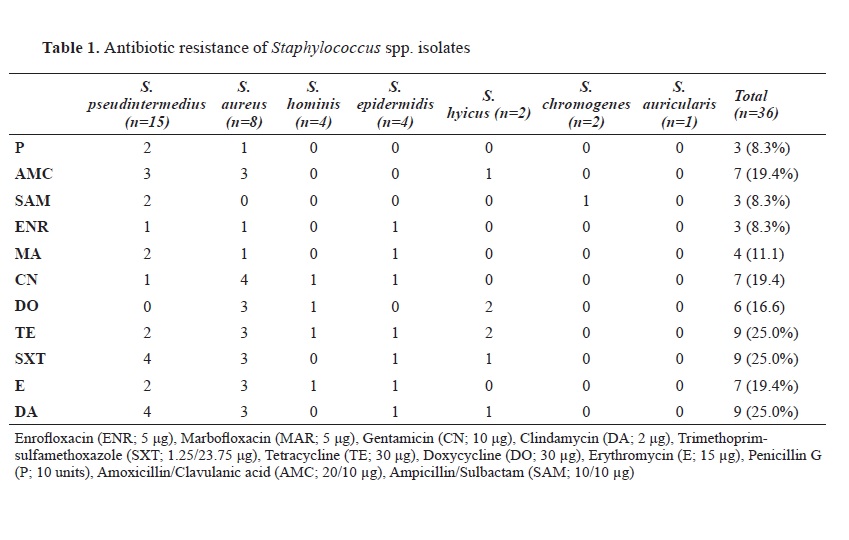
was found to be sensitive to all antibiotics. Aztreonam resistance was not detected in any of the isolates. No methicillin resistance was observed in any of the isolates. MDR was found in 30.5% of the strains.
Otitis is a common disease in dogs due to the anatomical features of the ear (
14, 15). The most commonly isolated bacterial agents from OE are Coagulase Positive
Staphylococcus spp. (CPS) and Coagulase Negative
Staphylococcus spp. (CNS) which are considered opportunistic agents (
16, 17). Numerous authors have investigated the presence of
Staphylococcus spp. in OE cases and have reported isolation rates between 36% and 60.3% (
7, 15). The current study had 36 (36%) dog OE isolates positive for Staphylococcus spp., similar to other reports (
7, 9, 18, 19, 20).
S. aureus in dog OE cases was reported between 9% and 55.76% (
7, 9, 18, 19, 21). In this study, 8 (22.2%) of 36
Staphylococcus spp. isolates were identified as
S. aureus.
S. pseudintermedius belongs to a community known as the
Staphylococcus intermedius group (SIG), which includes three different species,
S. pseudintermedius,
S. intermedius and
S. delphini. All members within the SIG group are known to colonize a large number of animal species.
S. pseudintermedius is a commensal in animals, especially in dogs. Canine isolates previously identified as
S. intermedius are currently referred to as
S. pseudintermedius unless proven otherwise by genomic studies.
S. intermedius isolation rates were reported between 13.6% and 73.9% in various studies (
19, 20, 22, 23). In the current study,
S. pseudintermedius was detected in 41.6% of the isolates. The genotypic methods for bacterial identification were considered more reliable rather than the phenotypic methods.
Members of the CNS group also play an important role in cases of otitis externa in dogs. Lilenbaum et al. (
19) reported that 60.3% of the isolates were CNS and 25% were
S. epidermidis. De Martino et al. (
20) identified 28%
S. chromogenes, 0.7% of
S. epidemidis, and 2.1%
S. hyicus in 143 isolates. Sarıerler and Kırkan (
21), Penna et al. (
7) and Borum et al. (
18) obtained CNS from their samples in 5.12%, 38%, and 17.3%, respectively.
Among the CNS isolates,
S. epidermidis,
S. chromogenes, and
S. hyicus stand out in several studies.
S. epidermidis has been reported in 25% (
19) and 11% (
7). In this study,
S. epidermidis was detected in 11.1%,
S. hyicus and
S. chromogenes in 5.5% of the samples, similar to other reports. However, De Martino et al. (
20) reported
S. epidermidis 0.7%,
S. hyicus 2.1%, and
S. chromogenes 28% in 143 samples. In another study conducted in our country, it was reported that CNS was detected in higher numbers than CPS (
9). In this study, CPS were found to have a higher prevalence than CNS. The differences in the reports might be due to geographical specificities, or dog age and breed.
Numerous studies have been examining the relationship between OE and breed characteristics. It has been reported that Cocker Spaniels, Poodle, Labrador Retrievers, German shepherd, and Fox Terriers are predisposed to OE due to structural differences in the ear canal and auricle (excess hair follicles, long and drooping structures), excess apocrine gland tissue, or excess amounts of cerumen (
15, 24, 25). The researchers found a higher rate of OE in dogs with floppy ears (73.2%) than in dogs with straight ears. The highest rate was obtained in Cocker Spaniels (26%) and Labradors (18%) which are lop-eared dogs, and in German Shepherds (14%) as straight-eared dogs (
26, 27, 28). In this study, when the number of samples and the isolation of
Staphylococcus species were compared, the presence of OE was found to be 80% in Terriers, 80% in Pomeranians, 66.6% in Rottweiler’s, 62.5% in Cocker Spaniels, 33.3% in Labradors, 25% in Golden Retrievers and 20.4% in mixed breeds. The highest isolation rate was from the mixed breeds (10/36, 27.7%) which were the most numerous sample group (49%).
The relationship between the presence of OE and age has also been demonstrated in many studies. Topala et al. (
24) reported the highest OE rate in dogs aged 5-8 years which was 33.4%. Kumar et al. (
27) reported that they detected the presence of OE mostly in dogs over the age of 3 (66.6%). Fernández et al. (
1) stated that OE was highest (43.4%) in dogs aged 2-5 years.
In the current study,
Staphylococcus spp. was detected in 9 (9/26, 34.6%) dogs aged ≤1 year, in 22 (22/63, 34.9%) aged 2-8 years, and in 5 (5/11, 45.4%) aged ≥9 years. These results were found to be statistically non-significant (p>0.05).
Comparing age and gender between different studies is difficult as they are defined differently (e.g., two age groups, young and old). Further studies are needed to establish an accurate correlation between these factors. The age can be additionally affected by other co-factors such as handling, hygienic conditions, geographical region, etc., which may define variable experimental or study conditions.
The researchers have demonstrated a relationship between gender and OE (
24, 26, 27). Kumar et al. (
27) stated that OE is more common in male animals than in females. On the contrary, other studies reported a higher rate in female dogs than in males (
1, 26). Topala et al. (24) reported 50.8% OE in female dogs, and they did not find a relationship between gender and OE. In the present study,
Staphylococcus spp. was detected in 15 (15/52, 28.8%) male and 21 (21/48, 43.75%) female dogs, but no significant relationship between gender and OE was observed (p>0.05).
Numerous studies aimed to investigate the relationship between OE and antimicrobial resistance (
21, 31). Infections caused by
Staphylococcus species are generally treated with antimicrobial agents that inhibit bacterial cell wall synthesis, especially β-lactams (
29). The researchers reported penicillin resistance between 30.7% and 72.5% (
21, 31). It was emphasized that the high level of resistance against penicillin was achieved due to β-lactamases produced by
S. aureus and
S. intermedius. Lyskova et al. (
2) found that 66% of
S. intermedius isolates were resistant to penicillin, but all of them were susceptible to amoxicillin/clavulanic acid (AMC). Kang et al. (
30) reported penicillin resistance in 92% of the
S. pseudintermedius isolates and AMC resistance in 6% of the samples. In other studies conducted in Türkiye, researchers reported that they did not detect AMC resistance in
S. aureus and CNS isolates (
22). Sığırcı et al. (
28) found penicillin resistance in 73.9%, AMC resistance in 22.2%, and ampicillin/sulbactam (SAM) resistance in 30% in
S. intermedius isolates. In addition, recent studies reported that 77.3% of
S. pseudintermedius isolates were resistant to penicillin and 6.8% to AMC resistance (
23). Bourely et al. (
10) reported penicillin resistance in 68.5% of
S. pseudintermedius isolates and 70.9% of
S. aureus isolates. In the present study, resistance to penicillin was found in 8.3%, AMC resistance in 19.4%, and SAM resistance in 8.3% of the isolates. The relatively low detection level of antimicrobial resistance compared to other studies was considered promising.
Fluoroquinolones obtained by adding fluorine at position six to the quinolone molecule, have a broad spectrum and are active against
Staphylococcus species. Lyskova et al. (
2) reported that they did not detect enrofloxacin resistance in all
Staphylococcus spp. isolates. Penna et al. (
7) found 48.6% of enrofloxacin resistance in
S. pseudintermedius isolates. Petrov et al. (
16) reported 32% resistance to enrofloxacin in CPS isolates. Öztürk et al. (
22) noted enrofloxacin resistance in 40% of the
S. aureus isolates and 11.1% in the CNS isolates. Sığırcı et al. (
28) found enrofloxacin resistance in 30.9% of the
S. intermedius isolates. In this study, enrofloxacin resistance was found in 8.3%, and marbofloxacin resistance at 11.1% of the isolates.
Amikacin and gentamicin aminoglycosides have been suggested for topical application in OE caused by Gram-positive bacteria (
20). The authors demonstrated that the gentamicin resistance ranged from 3% to 84.1% (
16, 19, 23, 28). Lyskova et al. (
2) reported that gentamicin resistance was not found in all
S. intermedius isolates. Penna et al. (
7) emphasised that gentamicin is more resistant than amikacin and stated that gentamicin resistance was 54.3% in
S. pseudintermedius isolates. In this study, gentamicin resistance was found in 19.4% of the isolates.
Tetracyclines, the first large group of antimicrobial agents, are among the most commonly used therapeutics in veterinary medicine (
32). It is known that the use of tetracycline with standard and inappropriate methods may lead to the transfer of resistance genes to recipient bacteria. The researchers revealed tetracycline resistance ranged from 7.69% to 94% (
23, 28, 30, 31). Petrov et al. (
16) found doxycycline resistance in 49% of KPS isolates. In this study, tetracycline resistance was found in 25% and doxycycline resistance in 16.6% of the isolates. It was considered promising that the determined resistance values were in the lower limits.
Sulfonamides are synthetic antibiotics ranked as ‘‘High priority’’ among veterinary drugs. The authors demonstrated resistance to trimethoprimsulfamethoxazole (SXT) between 15.38% and 88.8% (
22, 23, 30, 31). In this study, sulfonamide resistance was determined in 25% of the isolates.
Macrolide resistance is also a growing problem, probably proportional to the number of drugs used. The researchers reported erythromycin resistance from 23% to 80%. In another study conducted in Türkiye, this rate was 75% (
28). In the current study, erythromycin resistance was found in 19.4% and it was considered relatively lower compared to other studies.
The number of studies related to the lincosamide group, including lincomycin and clindamycin, is more limited. Oliviera et al. (
31) reported clindamycin resistance in 23.07% of the
S. intermedius isolates and 44.4% in the
S. aureus isolates. Lyskova et al. (
2) found 39% clindamycin resistance in
S. intermedius dog isolates with otitis media. In this study, clindamycin resistance was detected in 25% of the isolated, similar to other studies.
Methicillin resistance was not observed in this study, but further studies must be continuously made for regular updates.
MDR is defined as a resistance of a microorganism to multiple antimicrobial drugs which are structurally unrelated and have different molecular targets. Lilenbaum et al. (
20) reported that 90.9% of the isolates were resistant to at least one antibiotic, and MDR was detected in 36.4%. In the same study, it was noted that a single
S. haemolyticus isolate was resistant to all tested antimicrobials. Penna et al. (
7) emphasized that 89% of the 151 samples had multiple resistant isolates.
In this study, MDR was detected in 11 of 36 (30.5%)
Staphylococcus spp. isolates. If antimicrobial resistance can be transmitted from pets to humans, there would be significantly lower antimicrobial effectiveness in both animals and humans. The prevalence of antimicrobial resistance in pets (primarily dogs and cats) is of paramount importance in veterinary and human medicine, and therefore, continuous studies are necessary for up-to-date information.
Some limitations have to be considered in the present study. First, the number of dogs enrolled in this study was small. Second, data on clinical history and antimicrobial usage were not available. Third, the molecular diagnostic identification of
S. pseudintermedius was lacking. Finally, antimicrobial resistance genes were not included in the current study.
CONCLUSIONMH collected the samples, made the isolation and writing the article. AIK and BH performed isolation and phenotypic assessments. BBK designed the project, wrote the article, and executed the final approval of the version to be published.

 10.2478/macvetrev-2023-0012
10.2478/macvetrev-2023-0012
.jpg)