Abstract
This study aimed to investigate the effects of dietary supplementation with modified-hen-egg-yolk on plasma lipids and lipoprotein profiles in rats. During the four-week-experiment, 64 Wistar rats were divided into four groups of 16 (eight of both sexes), and fed commercial rat food (group C); food containing 70% commercial rat mixture and 30% freshly cooked egg yolk originating from laying hen eggs fed with 3% fish oil (group F); 3% palm olein (group P), or 3% lard (group L). The cooked egg yolk in the rat diet affected the concentrations of plasma total and LDL-cholesterol in males of the P and L groups. Cholesterol and total fat in the diet did not have a hypercholesterolemic effect on their own, but when in combination with fatty acid composition, they could contribute to an increase in plasma total and LDL cholesterol concentrations in rats. HDL-cholesterol was the most resilient plasma lipoprotein of rats to dietary treatments in our experiment. Compared to the control group, the addition of hen egg yolk to the rat diet regardless of its quality, adversely affected the values of HDL-C/TC and HDL-C/LDL-C in both males and females.
Keywords: egg yolk, plasma lipids, rat, dietary supplementation, hen
INTRODUCTION
Hyperlipidemia is generally considered one of the major risk factors for atherosclerosis and coronary heart disease (
1). Most of the evidence relates specifically to hypercholesterolemia, but hypertriglyceridemia may also play a significant role, although not to the same extent as hypercholesterolemia do. Many studies have shown an almost linear correlation between the levels of total plasma cholesterol or low-density lipoprotein (LDL) and the stage of atherosclerosis and ischemic heart disease (
2, 3). It is also important to note the negative correlation between symptomatic atherosclerosis and high-density lipoprotein (HDL) levels. Nutrition is considered to be the prime factor in lowering serum LDL and raising HDL levels. Abundant intake of saturated animal fats in diets increases the level of serum cholesterol (
4). In contrast, a diet in which saturated fats are replaced by monounsaturated or polyunsaturated fats lowers LDL, and in some cases simultaneously lowers HDL levels moderately (
5). Polyunsaturated fatty acids (PUFAs) of the n-3 series, which are mainly obtained from fish and fish oil, have a clear hypolipidemic effect (
6). There is an inverse effect of saturated (SFA) and polyunsaturated fatty acids on plasma cholesterol. Based on these findings, many nutritionists conclude that the key determinant of serum cholesterol levels is the ratio between polyunsaturated and saturated fatty acids in the diet (
7). Both epidemiological and clinical studies have shown a reduction in coronary heart disease mortality in populations consuming even relatively small amounts of n-3 polyunsaturated fatty acids over an extended period (
8), but the end effect also depends on the ratio of n-6/n-3 polyunsaturated fatty acids (
9). In Western countries, more than 60% of total lipids, 70% of saturated fats, and 100% of food cholesterol originate from animal products (
10). The consumer preferences for animal products are likely to continue. Therefore, in the prevention of coronary heart disease, it would be of strategic interest to design and/or modify animal products so that the dietary risks of coronary heart disease are minimized. Wild animal and fish meat, hen eggs produced under completely natural conditions, and wild plants contain higher amounts of n-3 fatty acids compared to domesticated animals’ meat and cultivated plants (
11). The composition of meat, fish meat, and eggs depends on the food that these animals eat. Fishmeal, flax, and n-3 fatty acids of algae added to the diet of laying hens increase the content of n-3 polyunsaturated fatty acids in egg yolk and provide the market with eggs enriched with n-3 polyunsaturated fatty acids (
4). Approximately, three such eggs provide the same amount of n-3 polyunsaturated fatty acids as one meal of fish (
12). Thus, hen eggs enriched with n-3 polyunsaturated fatty acids could be considered as a source of these compounds that would suffice the daily intake according to the United Nations Food and Agriculture Organization recommendations (FAO): ranges for adults for linoleic acid (LA) - 2.5–9% of energy, total n-3 PUFAs - 0.5–2% of energy, and EPA (eicosapentaenoic acid) + DHA (docosahexaenoic acid) - 250 to 2000 mg/d (
13). The leading advantage of providing n-3 PUFAs through hen eggs in comparison to fish is not only that eggs are a cheaper and more complete source of essential nutrients, but also that laying hens act as “biological sieves” in terms of removal or reduction of any undesirable contaminant. In addition, the fatty acid composition of egg yolk lipids can be successfully altered through the diets of laying hens (
14). Moreover, eggs are a widespread animal product available for people’s consumption without any restrictions (
15). Hence, there is a great interest in altering the cholesterogenic properties of (hen) egg yolk (
16). The dietary fish oil, palm olein, and lard can influence the lipid composition of laying hen egg yolk. The supplementation of a modified-hen-eggyolk-lipid-composition diet can affect plasma lipids in rats, depending on sex.
We aimed to investigate the effect of a modifiedhen- egg-yolk-lipid-composition diet and sex on plasma lipid concentrations and ratios in rats.
MATERIAL AND METHODS
Animals and experimental design
In the first phase of the study, hen egg yolks with a specified quality were produced. Lohman Brown laying hens (n=90) were at 34 weeks of production and 56 weeks of age. They were kept under standard conditions of industrial production. The hens were randomly allocated into three groups – HF, HP, and HL. The HF group feed mixture contained 3% fish oil (“Henry Lamotte” GMBH, Bremen, Germany), the HP group feed mixture contained 3% palm olein (“Alami Corporation SDN, BHD”, Selangor, Malaysia), and the HL group feed mixture contained 3% lard (“Meat Industry Bosanska Gradiška”, Bosanska Gradiška, BiH). The eggs which were used for the rat diet preparation were collected in the fifth and sixth weeks of egg production. The hen egg yolks were analyzed for total lipids, triglycerides, cholesterol, and fatty acid composition. The results of these analyzes have been presented earlier (
17).
The experimental part was conducted over four weeks. Wistar rats (n=64), 32 females, and 32 males at the age of four months were used. All experiments were performed according to the guidelines established by the European Community for the Care and Use of Laboratory Animals, Ethics and Animal Welfare Committee of the International Council for Laboratory Animal Science. This study was conducted in accordance with the Law of Protection and Welfare of Animals, (“Sl. glasnik BiH”, no. 25/2009 and 9/2018); and approved by the Ethical Committee of the University of Sarajevo - Veterinary Faculty (Approval no: 07-03-967-2/21).
Rat diets
Animals were fed ad libitum from the beginning to the end of the experiment. Three days before the start of the experiment, all rats were fed with a commercial pelleted feed mixture (“MB-MIX”, Banja Luka, BiH). After three days of adaptation to the experimental housing conditions, the animals were weighed and randomly divided into four groups - C, F, P, and L, with 16 individuals - eight individuals of both sexes, taking into account the mean values of the initial sex weights within groups to be approximated and without statistically significant differences. The experimental diets contained 70% of a commercial feed mixture for rats and 30% of freshly cooked egg yolk originating from HF, HP, and HL groups. The eggs were boiled for 15 min, cooled, and the yolk was separated and homogenized in a mortar. The commercial feed mixture was immersed in the same amount of water and mixed with the egg yolk. The resulting mass was manually homogenized, dried for 24 h at room temperature, and then processed in an oven at 50 °C to a constant weight. The finished product was stored in paper bags in a dry and dark place.
The control group (C) of rats was fed with a commercial pelleted feed mixture, the F group with a commercial feed mixture supplemented with 30% HF egg yolk, the P group with a commercial feed mixture supplemented with 30% HP yolk, and the L group with a commercial feed mixture supplemented with 30% HL egg yolk. The food was offered to the animals every second day. Food consumption and weight gain were measured weekly. The composition of rat diets and their fatty acid composition are given in Tables 1 and 2, respectively, previously reported elsewhere (
18). The experimental diets had higher total fat content due to the supplementation of 30% egg yolk. This increase was largely at the expense of nitrogen-free extract (NFE) and fiber (
Table 1). The experimental diets had higher saturated-stearic and palmitic acids, higher monounsaturated oleic and palmitoleic acids, and significantly lower linoleic acid content compared to the control group diet (
Table 2).
.jpg)
.jpg)
Sample preparation and analysis
After 28 days of experimental feeding, the rats were subjected to 12 h fasting before being sacrificed. The next day they were weighed and the blood was sampled from the abdominal aorta under anesthesia with diethyl ether. The blood was collected in 3 ml EDTA vacutainers. The plasma was separated by centrifugation immediately after sampling at 3,000 rpm for 10 min. The plasma triglyceride concentration was determined by the enzymatic GPO-PAP method by using a lipid-purifying factor test („Human“, Wiesbaden, Germany) and a standard of 200 mg/dl. Total plasma cholesterol was also measured enzymatically by the GODPAP method (“Semikem” test, Sarajevo, BiH) with a standard of 200 mg/dl. Plasma HDL-cholesterol was analyzed by a semi-micro determination test for total cholesterol after previous precipitation and centrifugation (“Human”, Wiesbaden, Germany). A standard of 50 mg/dl was used for the calculation. The concentration of plasma LDL-cholesterol was calculated from the concentration of plasma total cholesterol, HDL-cholesterol and triglycerides by the Friedewald formula (
19). Total plasma lipids were determined by the colorimetric method with vanillin (“Semikem”, Sarajevo, BiH) with a standard of 10.8 g/L.
Statistical analysis
The data were processed by a two-factor analysis of variance - ANOVA. Differences in the obtained treatment mean values were further analyzed by Duncan’s multiple tests if the analysis of variance showed a statistically significant treatment effect (P<0.05).
RESULTS
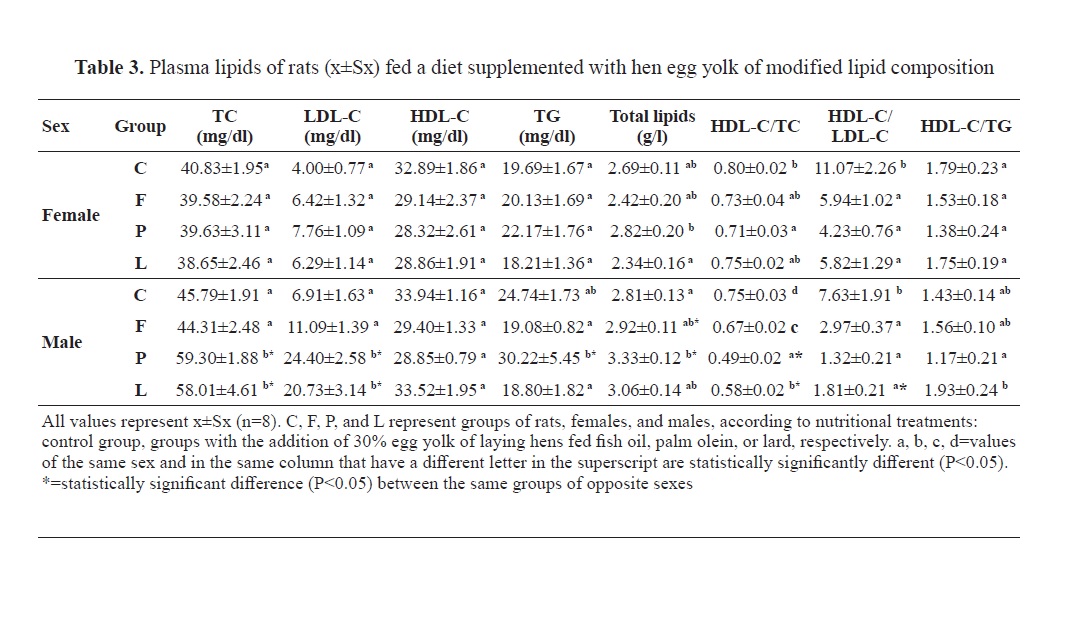
Blood plasma lipids in rats fed a diet with added hen egg yolks of modified lipid contents are presented in
Table 3, while
Fig. 1 and
Fig. 2 show the changes in concentrations and ratios of measured plasma lipids of the experimental groups (females and males) expressed as a percentage of change relative to the control group of rats (100%) not shown in the graphs.
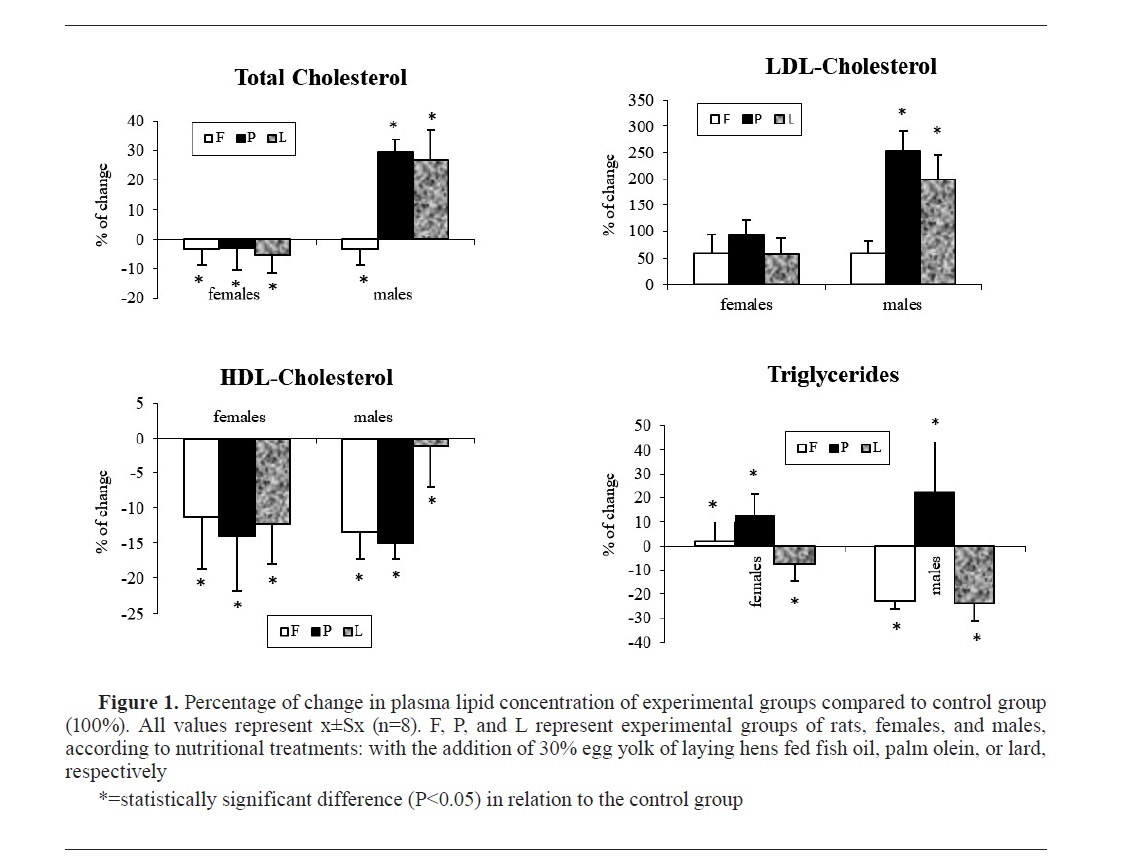
 DISCUSSIONTotal and LDL-cholesterol
DISCUSSIONTotal and LDL-cholesterol
Our results showed almost identical patterns of changes in plasma total and LDL-cholesterol in rats fed with a diet supplemented with hen egg yolk (
Table 3). But there was an obvious difference in the values of these two parameters in rats of different sexes. In the case of males, dietary supplementation with egg yolks derived from laying hens fed palm olein and lard has significantly increased plasma total and LDL-cholesterol compared to the rats fed with commercial feed mixture and an experimental diet supplemented with egg yolk from laying hens fed with fish oil. This should not be solely related to the cholesterol content in the rat diets (
Table 1) considering reports from other studies (
20). Another argument and possible confirmation of this claim is the absence of a significant difference in the values of plasma total and LDL-cholesterol between groups C and F when the diet of the control group contained 10 times less cholesterol than the diet of group F; moreover, the diet of group F had the highest cholesterol content among all experimental rat diets (
Table 1). Therefore, our results rather support the thesis of the suppressive effect of dietary cholesterol on its endogenous biosynthesis and the consequent reduction of plasma cholesterol levels (
21). The effect of dietary cholesterol on blood cholesterol is truly questionable (
22). The interpretation of food-disease relationship data should be done very carefully (
23), and when it comes to egg yolk added to food, it is necessary to take into account the amount of total dietary intake, and especially its quality in terms of content, not only of cholesterol and SFAs and PUFAs (
24) but also of various micronutrients (
25). It is obvious that cholesterol derived from hen egg yolk cannot be the only key determinant of the cholesterol properties of hen egg yolk (
21). The fatty acid composition of egg yolk lipids plays a significant role. Palmitic acid is the main saturate of animal fats, but it is also found in vegetable oils, and together with myristic and lauric acid, they are considered hypercholesterolemic. The highest amount of palmitic acid in our experiment was found in the rat diets of group P followed by the diet of group L, and group F, while the diet of the control group had the lowest content of this fatty acid (
Table 2). Judging by the differences in the concentrations of plasma total and LDL-cholesterol among rat groups, it seemed that the palmitic acid of the diet could be the responsible hypercholesterolemic component. There was an opposite significant (P<0.05) change in the concentration of total cholesterol in all experimental groups compared to the control (
Fig. 1). Females, as more resistant responders, showed negative changes compared to the control. Compared to palmitic, stearic acid is hypocholesterolemic, but compared to LA, it is hypercholesterolemic (
26). Judging by the content of stearic acid in rat diets that we used in our experiment (
Table 2), this thesis seems very probable to us. The most common fatty acid in rat diets with the addition of egg yolk was oleic. The highest content of oleic acid was in the diets of P and L rats, which also had the highest content of palmitic acid (
Table 2). The oleic/palmitic acid ratio was about 2:1. Following the finding that male P and L rats fed with a high palmitic and oleic acid content diet had significantly higher total and LDL-cholesterol compared to the C and F groups (
Table 3), it was indicative that there was a “neutral” effect of dietary oleic acid on plasma total and LDL-cholesterol, or probably, there was a large difference between these two fatty acids in their potency of altering total and LDL-cholesterol in plasma, which certainly should not be ruled out.
The absence of statistical significance of differences in the concentrations of plasma total and LDL-cholesterol in rats of group F compared to controls could be due to the SFA content (mainly palmitic and stearic acid) which was 26.87%, 31.22%, and 30.73% in F, P and L diets, respectively (
Table 2). If the F group diet is considered as high in n-3 PUFA content, it could be attributed to the lower plasma total and LDL-cholesterol due to its hypocholesterolemic effects (
27).
HDL-cholesterolContrary to the findings of Aguila (
27), HDL-cholesterol concentration proved to be a stable plasma lipoprotein of the current study. Its concentration appeared to be most dependent on a combination of multiple lipid food components. Farrell (
28) found that a diet containing two eggs per day significantly reduced HDL in humans, even when consuming eggs of laying hens that were fed with a mixture of fish or linseed oil (7% each). In our experiment, group F rats also had lower HDL cholesterol, but this reduction was not statistically significant (
Table 3). Similar values of HDL-cholesterol were gained by a diet with low cholesterol content and relatively high content of LA in group C, and by a diet with 10 times more cholesterol and with twice lower content of LA in group L (
Tables 1,
2, and
3). There may be an interaction between dietary PUFAs and cholesterol that can affect HDL metabolism in different ways (
29). A diet rich in LA (C) and a diet with high content of oleic acid (L) (
Table 2) resulted in similar HDL values in rats (
Table 3), despite large differences in saturates content. This is in compliance with the findings of Dreon et al. (
30) who found no differences in HDL-cholesterol concentration between polyunsaturated and monounsaturated fatty acids in food. However, this was most likely not a pure effect of the oleic acid which was with high content in both the L and P group. The latter had lower HDL-cholesterol compared to the C and L groups (
Table 3).
TriglyceridesHigh-fat diets, such as those consumed by animals in the experimental groups (
Table 1), compared to the high-carbohydrate diets fed to the control group, generally do not increase serum triglyceride levels (
Table 3) unless there are some lipid disorders (
26). Dietary SFAs do not generally increase serum triglycerides as they do with cholesterol. The highest concentration of triglycerides was found in rats of group P (
Table 3) whose diet had the highest total content of SFAs (31.82%) and the lowest content of total fatty acids (83.29%). Long-chain saturates, such as palmitic acid, most present in group P rat diet, do not increase serum triglycerides compared to monounsaturated fatty acids (
26). In comparison to n-6, the n-3 PUFAs, especially those with very long chains such as EPA and DHA, have a greater effect on triglyceride metabolism. If the laying hen eggs of group HF, fed with fish oil, are considered as n-3 enriched eggs, then hypotriglyceridemia that was found in males of group F compared to other groups, was an expected finding. Many studies showed that the most consistent and preferential effect of n-3 PUFAs on plasma lipids is a reduction in triglyceride levels (
31), which appears to be dosedependent.
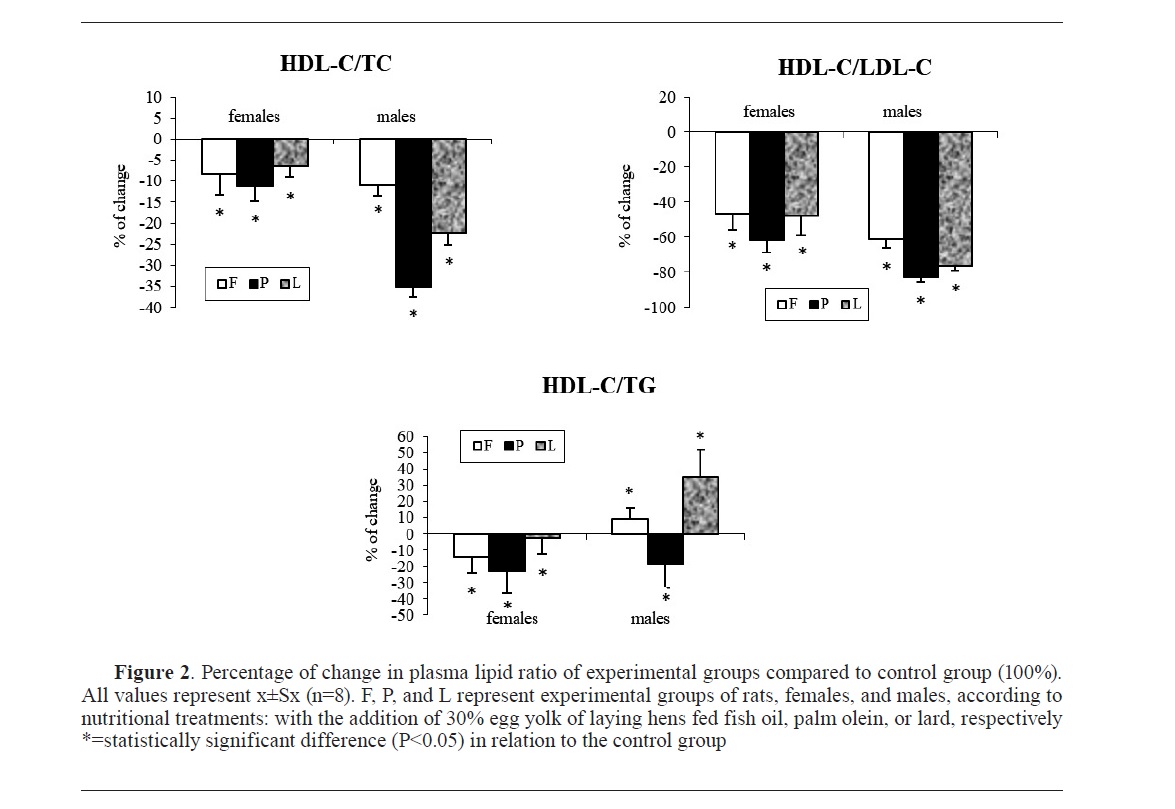
The ratio of plasma lipoprotein profilesIn the HDL-C/LDL-C ratio, the addition of hen egg yolk to the rat diet led to a statistically significant reduction in this ratio in all experimental groups compared to the control, regardless of the quality of the added egg yolk (
Fig. 2). Thus, the properties of fat in the laying hen diet affected the relative amount of cholesterol carried by the HDL fraction in rats, but not the relative presence of cholesterol transported by the desirable or undesirable lipoprotein fraction (
32).
CONCLUSION
Egg yolks from laying hens fed with 3% palm olein and lard feed mixtures had approximately the same effect on the increase of total plasma cholesterol in rats. The lipid component from the diet with the most potent effect on the increase in total and LDL-cholesterol was palmitic acid, while oleic and stearic were mostly “neutral”. Dietary cholesterol and total fat did not have a hypercholesterolemic effect on their own, but in combination with the fatty acid composition, they could contribute to an increase in plasma total and LDL cholesterol in rats. These effects are related to males while females did not respond to the same treatment. The addition of hen egg yolk to the rat diet, regardless of its quality, adversely affected HDL-C/TC and HDL-C/LDL-C values in both males and females, which can be considered unfavorable from the consumer’s point of view. One of the alternative ways to change the cholesterol properties of laying hen egg yolk can be diet alteration. Fish oil has proven to be the most potent positive dietary modulator in our experiment.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTS
The authors would like to express gratitude to: Brovis d.d. Visoko, Bosnia and Herzegovina, the Norwegian University of Life Sciences, the Department of Poultry and the Department of Physiology of the University of Sarajevo – Veterinary Faculty, and prof.dr. Milan Baltić from the Faculty of Veterinary Medicine, University of Belgrade, for the support regarding the experimental and laboratory procedures.
AUTHORS’ CONTRIBUTIONS
AH, MH, JK and AG conceived and supervised this study. AH, HO and EPH completed the main experimental content. AHP and AA collected and analyzed data. AH, HO and AS wrote the first draft of the manuscript. All authors contributed to the critical revision of the manuscript and have read and approved the final version.
References
1.
Isdadiyanto, S., Sitasiwi, A.J., Mardiati, S.M. (2020). The lipid profile of rats (Rattus norvegicus L.) induced by high fat ration after exposed to ethanolic neem (Azadirazchta indica) leaf extract. J Phys Conf Ser. 1524, 012126.
https://doi.org/10.1088/1742-6596/1524/1/0121262. Bogoriani, N.W., Ariati, N.K. (2018). The activity of Bali Andong rhizome extract of Cordyline terminalis Kunth as a hypolipidemic agent in Wistar rats with high-cholesterol diets. eIJPPR 8(1): 75-80.
3. Wu, J.H., Micha, R., Mozaffarian, D. (2019). Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 16(10): 581-601.
https://doi.org/10.1038/s41569-019-0206-1 PMid:31097791
4. Siri-Tarino, P.W., Sun, Q., Hu, F.B., Krauss, R.M. (2010). Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 91(3): 502-509.
https://doi.org/10.3945/ajcn.2008.26285 PMid:20089734 PMCid:PMC2824150
5. Bos, M.B., de Vriesa, J.H.M., Feskensa, E.J.M., van Dijka, S.J., Hoelenc, D.W.M., Siebelinka, E., Heijligenbergc, R., de Groot, L.C.P.G.M. (2010). Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis. 20(8): 591-598.
https://doi.org/10.1016/j.numecd.2009.05.008 PMid:19692213
6. Kromhout, D., de Goede, J. (2014). Update on cardiometabolic health effects of ω-3 fatty acids. Curr Opin Lipidol. 25(1): 85-90.
https://doi.org/10.1097/MOL.0000000000000041 PMid:24345990
7. Siri-Tarino, P.W., Sun, Q., Hu, F.B., Krauss, R.M. (2010). Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 12(6): 384-390.
https://doi.org/10.1007/s11883-010-0131-6 PMid:20711693 PMCid:PMC2943062
8. Kapoor, B., Kapoor, D., Gautam, S., Singh, R., Bhardwaj, S. (2021). Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr Nutr Rep. 10(3): 232-242.
https://doi.org/10.1007/s13668-021-00363-3 PMid:34255301
9. Saini, R.K., Keum, Y.S. (2018). Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - a review. Life Sci. 203, 255-267.
https://doi.org/10.1016/j.lfs.2018.04.049 PMid:29715470
10. Jiang, Z., Sim, J.S. (1993). Consumption of n-3 polyunsaturated fatty acid-enriched eggs and changes in plasma lipids of human subjects. Nutrition 9(6): 513-518.
11. Simopoulos, A.P. (1999). New products from the agri-food industry: the return of n-3 fatty acids into the food supply. Lipids 34(S1Part3): S297-S301.
https://doi.org/10.1007/BF02562324 PMid:10419184
12. Lewis, N.M., Seburg, S., Flanagan, N.L. (2000). Enriched eggs as a source of n-3 polyunsaturated fatty acids for humans. Poult Sci. 79(7): 971-974.
https://doi.org/10.1093/ps/79.7.971 PMid:10901195
13. FAO (2010). Fats and fatty acids in human nutrition. Report of an expert consultation. FAO FAO Food Nutr Pap. 91, 1-166.
14. Franczyk-Zarow, M., Kostogrys, R., Szymczyk, B., Jawien, J., Gajda, M., Cichocki, T., Wojnar, L., Chlopicki, S., Pisulewski, P. (2008). Functional effects of eggs, naturally enriched with conjugated linoleic acid, on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice (apoE/ LDLR−/−). Br J Nutr. 99(1): 49-58.
https://doi.org/10.1017/S0007114507793893 PMid:17678565
15. Airaodion, A.I., Ngwogu, A.C., Ekenjoku, J.A., Ngwogu, K.O. (2019). Egg yolk causes high blood pressure in Wistar rats. J BioSci Biotechnol. 11(11): 94-99.
16. Bogoriani, N.W., Putra, A.A.B., Heltyani, W.E. (2019). The effect of intake duck egg yolk on body weight, lipids profile and atherosclerosis diseases in male wistar rats. IJPSR 10(2): 926-932.
17. Hodzic, A., Hamamdzic, M., Gagic, A., Mihaljevic, M., Vegara, M., Krnic, J., Pasic-Juhas, E. (2008). The influence of dietary palm olein, fish oil and lard on the egg yolk and plasma lipid composition and performances of laying hens. Pol J Vet Sci. 11(1): 1-7.
18. Hodzic, A., Hamamdzic, M., Gagic, A., Crnkic, C., Kadric, M., Pasic-Juhas, E., Krnic, J., Hrkovic, A. (2012). Lipid composition of liver in rats fed diets supplemented with egg yolks of modified composition. Acta Vet. 62 (4): 455-466.
https://doi.org/10.2298/AVB1204455H 19 .Friedewald, W.T., Levy, R.I., Fredrickson, D.S. (1972). Estimation of the concentration of lowdensity lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clin Chem. 18(6): 499-502.
https://doi.org/10.1093/clinchem/18.6.499 PMid:4337382
20. Adamopoulos, P.N., Papamichael, C.M., Zampelas, A., Moulopoulos, S.D. (1996). Cholesterol and unsaturated fat diets influence lipid and glucose concentrations in rats. Comp Biochem Physiol B Biochem Mol Biol. 113 (3): 659-663.
https://doi.org/10.1016/0305-0491(95)02078-0 PMid:8829815
21. Yu, Z., Mao, C., Fu, X., Ma, M. (2019). High density lipoprotein from egg yolk (EYHDL) improves dyslipidemia by mediating fatty acids metabolism in high fat diet-induced obese mice. Food Sci Anim Resour. 39(2): 179-196.
https://doi.org/10.5851/kosfa.2018.e38 PMid:31149661 PMCid:PMC6533406
22. Yang, F., Ma, M., Xu, J., Yu, X., Qiu, N. (2012). An egg-enriched diet attenuates plasma lipids and mediates cholesterol metabolism of high-cholesterol fed rats. Lipids 47(3): 269-277.
https://doi.org/10.1007/s11745-011-3646-x PMid:22234516
23. Liu, A.G., Ford, N.A., Hu, F.B., Zelman, K.M., Mozaffarian, D., Kris-Etherton, P.M. (2017). A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr J. 16, 53.
24. Chen, X., Du, Y., Boni, G.F., Liu, X., Kuanga, Genga, Z. (2019). Consuming egg yolk decreases body weight and increases serum HDL and brain expression of TrkB in male SD rats. J Sci Food Agric. 99(8): 3879-3885.
25. Fernandez-Robredo, P., Rodriguez, J.A., Sadabaa, L.M., Recaldea, S., Garcia-Layana, A. (2008). Egg yolk improves lipid profile, lipid peroxidation and retinal abnormalities in a murine model of genetic hypercholesterolemia. J Nutr Biochem. 19(1): 40-48.
https://doi.org/10.1016/j.jnutbio.2006.12.020 PMid:17531457
27. Aguila, M.B., Loureiro, C.C., Pinheiro, A.R., Mandarim-de-Lacerda, C.A. (2002). Lipid metabolism in rats fed diets containing different types of lipids. Arq Bras Cardiol. 78(1): 32-38.
https://doi.org/10.1590/S0066-782X2002000100003 PMid:11826345
28. Farrell, D.J. (1993). Enrichment of hen eggs with n-3 long-chain fatty acids and evaluation of enriched eggs in humans. Am J Clin Nutr. 68(3): 538-544.
https://doi.org/10.1093/ajcn/68.3.538 PMid:9734728
29. Yashodhara, B.M., Umakanth, S., Pappachan, J.M., Bhat, S.K., Kamath, R., Choo, B.H. (2009). Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad Med J. 85(1000): 84-90.
https://doi.org/10.1136/pgmj.2008.073338 PMid:19329703
30. Dreon, D.M., Vranizan, K.M., Krauss, R.M., Austin, M.A., Wood, P.D. (1990). The effect of polyunsaturated fat vs monounsaturated fat on plasma lipoproteins. JAMA 263(18): 2462-2466.
https://doi.org/10.1001/jama.263.18.2462 PMid:2329634
31. Harris, W.S., Dayspring, T.D., Moran, T.J. (2013). Omega-3 fatty acids and cardiovascular disease: new developments and applications. Postgrad Med. 125(6): 100-113.
https://doi.org/10.3810/pgm.2013.11.2717 PMid:24200766
32.
Kuang, H., Yang, F., Zhang, Y., Wang, T., Chen, G. (2018). The impact of egg nutrient composition and its consumption on cholesterol homeostasis. Cholesterol 2018: 6303810. https://doi.org/10.1155/2018/6303810 PMid:30210871 PMCid:PMC6126094

 10.2478/macvetrev-2023-0013
10.2478/macvetrev-2023-0013
.jpg)
.jpg)


