Mycoplasma species cause mastitis, arthritis, genital infections, otitis, keratoconjunctivitis, abortion, and death in cattle. Although antibiotics such as oxytetracycline, tilmicosin and spectinomycin are frequently used in the field, it has been reported that some Mycoplasma species are now resistant to these antibiotics. (
1). The agent, which is abundant in the secrete from the lungs and noses of patients and carriers, can be directly transmitted by aerogenic transmission. Predisposing factors include poor care or feeding, cold, stuffy environment and stress. Disease clinical signs include difficulty in breathing, nasal discharge, cough and loss of efficiency (
2).
The major circulating form of vitamin D, calcifediol (25(OH)D
3) and its active form calcitriol (1,25(OH)
2D
3) were originally considered steroid hormones important in calcium homeostasis for bone health. However, recent studies have shown that 25(OH)D
3 also plays a role in endothelial function, cell proliferation and immunity. 1,25(OH)
2D
3 regulates the expression of genes encoding antimicrobial peptides and cathelicidins (
3). Moreover, vitamin D deficiencies have been associated with increased incidence of lower respiratory tract infections. Vitamin D appears to modulate the immune response in many infections, including respiratory tract infections. Various studies have investigated the minimum concentration of 25(OH)D
3 required for optimal immune function in cattle. The findings suggest that concentrations below 30 ng/mL represent vitamin D deficiency (
4).
Vitamin A, which is very important for organismal health refers to a group of fat-soluble retinoids, including retinol, retinal and retinyl esters (
5). Vitamin A is stored in the liver for release into the bloodstream as retinol when needed. It plays a role in many important tasks, such as growth, vision, bone development, differentiation of epithelial tissue and preservation of its integrity. More specifically, vitamin A stimulates the differentiation, reproduction and secretion of mucus-secreting cells in epithelial tissue. Vitamin A deficiency leads to proliferation, scarification, and keratinization in epithelial basement cells, which are particularly noticeable in respiratory, eye, conjunctival, salivary, reproductive and urinary tract epithelia (
6). McGill et al. (
5) investigated the effect of bovine respiratory syncytial virus vaccine on the immune response in calves with vitamin A deficiency. They reported that deficient calves did not respond to the vaccine or gain protection while their inflammatory responses in the infected lung were abnormal. This suggests that vitamin A is an important immunity regulator in the respiratory mucosa in the calf model they used.
The present study aimed to determine the clinical manifestations and laboratory findings of cattle naturally infected with
M. bovis and to determine serum vitamin A and 25(OH)D
3 levels. Defining the disease clinically and determining vitamin A levels, associated with the respiratory system epithelium and 25(OH)D
3 levels, which are important for immunity, in the presence of
M. bovis can contribute to improved prophylaxis and treatment of pneumonia in cattle, which causes serious economic losses for cattle production.
MATERIAL AND METHODSAnimals and study designAnimals included in the study are part of 103 cattle from 21 different farm, different breed (64 Holstein, 39 Simental) and showing pneumonia clinical signs (cough, fever, nasal discharge, respiratory distress) and a control group of 10 healthy cattle from a tuberculosis-free (non-exposed unvaccinated and/or unchallenged). The sampled animals were aged 1-4 years from various districts of Izmir province in western Türkiye. Quantitative clinical evaluations (general appearance, respiratory signs, nasal discharge, cough severity, arthritis, mastitis) and clinical examinations (respiratory frequency, heart rate, rectal temperature) were performed on all 113 cattle. Amies transport media with charcoal (Transwab Pernasal, MW173C, Medical Wire & Equip. Co. Ltd, UK) were used for collecting nasal swab samples. These were sent to Firat University, Faculty of Veterinary Medicine, Department of Microbiology for agent isolation under appropriate transport conditions. Samples were stored at -20 °C until sowing. Before starting the study, ethical approval was obtained from Dokuz Eylül University Animal Experiments Local Ethics Committee, (Decision no. 15/2021 from 24.03.2021). Informed consent has been obtained for client-owned animals included in this study.
Mycoplasma isolation and molecular characterizationOne hundred and thirteen nasal swab samples were collected from clinically infected cattle in May-August 2021 for
M. bovis isolation. Each sample was transferred to a liquid medium containing 6 ml of pleuro pneumonia like organism (PPLO) and incubated at 37 ºC in 5% CO
2 for 3-4 days. After incubation, liquid cultures were filtered through a 0.45 μm filter to avoid the risk of contamination. The filtrates were diluted to 10-4 and the samples were incubated in 5% CO
2 for 5 days. Diluted PPLO liquid cultures were transferred to modified hayflick agar with turbidity observed in the last tubes and colony formation was monitored for 7 days. Colonies on the agar surface were examined under a stereomicroscope for a typical “fried egg” appearance. Single colonies on the agar surface were cut with a scalpel and transferred to PPLO broth to obtain pure culture. This procedure was repeated at least three times. Colonies were then transferred to sterile tubes containing 50% mycoplasma broth and 50% horse serum and stored in a deep freezer at -80 °C for genomic DNA extraction. DNA of suspected
M. bovis isolates was extracted by phenol chloroform method. Target DNAs were first validated with primers, to the Mycoplasma genus and then analyzed by PCR using spesific primers for
M. bovis, M. alkalescens, M. arginini, M. dispar, M. bovirhinis and M. canis (
Table 1).
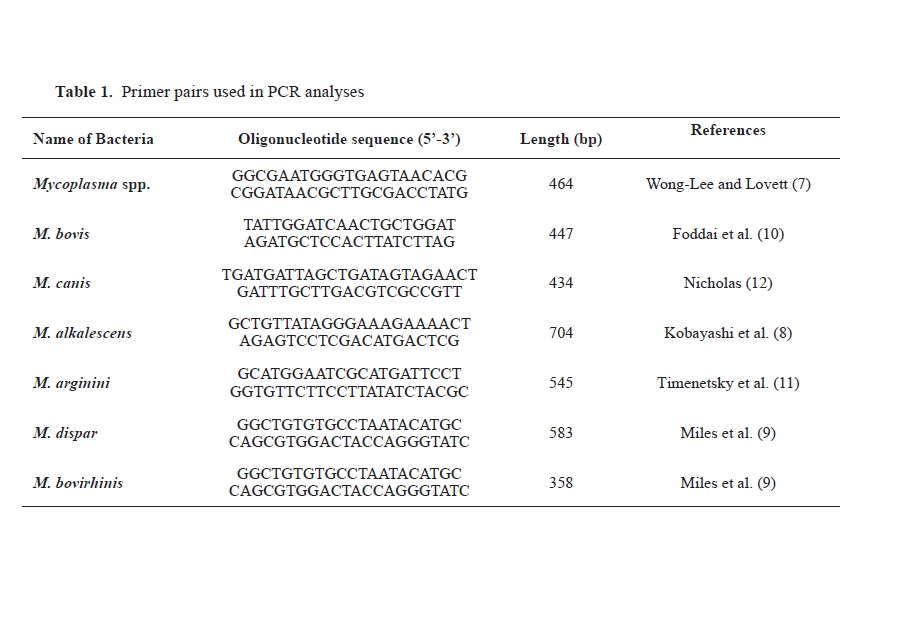
PCR amplification was then performed using separate protocols for each genomic DNA. DNAs belonging to mycoplasma species previously isolated in our laboratory were used as PCR positive controls. The PCR was performed in a Techne TC-512 gradient thermal cycler (Techne, UK) in a total reaction volume of 50 μl containing 5 μl of 10 x PCR buffer (750 mM Tris-HCl, pH 8.8, 200 mM (NH4)
2SO
4, (0.1% Tween-20), 5 μl of 25 mM MgCl, 5 μl of 250 μM of each deoxynucleotide triphosphate, 0.25 μl of 1.25 U Taq DNA polymerase (MBI, Fermentas), 2.5 μl of 20 pmol of each primer, 5 μl of template DNA. 16sRNA PCR reaction condition for Mycoplasma species were as follows: initial denaturation at 95 °C for 5 min, denaturation at 95 °C 30 s annealing at 57 °C for 30 s and extension at 72 °C for 30 s for 35 cycles, followed by a final extension step at 72 °C for 5 min. Amplified products were detected by staining with 10 mg/ml ethidium bromide after electrophoresis at 80 V for 2 h in 2% agarose gels. Finally, the agarose gel electrophoresis results of PCR products were evaluated (
7, 8, 9, 10, 11, 12). Moreover, the obtained PCR positive products were confirmed to be
M. bovis by performing sequencing analysis.
Blood sample collection and biochemistryFive ml blood was collected from the V. jugularis of each cow in yellow-capped (BD Vacutainer® SST™II Advance) tubes. After the blood samples were centrifuged (Nuve, NF 800R, Türkiye) at 3000 g for 10 minutes, the serum samples were stored at -20 °C until analysis. Serum biochemistry analysis was conducted using a Fuji Dri-Chem NX500i analyzer (Fujifilm Corporation®, Tokyo, Japan). A comprehensive measurement panel was made of albumin (ALB), alanine aminotransferase (ALT), urea nitrogen (BUN), cholesterol (CHOL), creatinine (CRE), gamma-glutamyl transpeptidase (GGT), glucose (GLU), total bilirubin (TBIL), total protein (TP) and alkaline phosphatase (ALP).
Evaluation of vitamin profileFor 25(OH)D
3 detection, a high-performance liquid for vitamin A analysis from a serum samples chromatography (HPLC) device (Shimadzu OGU-20A, Japan) and Immunoassay device (Siemens AdviaCentaur XP, Germany) were used.
Statistical analysisStatistical analyses were performed using the SPSS 22 program. First, normality tests were performed to determine whether the variables were normally distributed based on the coefficients of variation, Shapiro-Wilk values and histograms. Student’s t tests were used for the normally distributed clinical findings, biochemical parameters and vitamin parameters. The result was considered statistically significant if the p value was less than 0.05.
RESULTSThe PCR analysis of nasal swab samples obtained from 24 cattle with
M. bovis pneumonia is presented in
Fig. 1.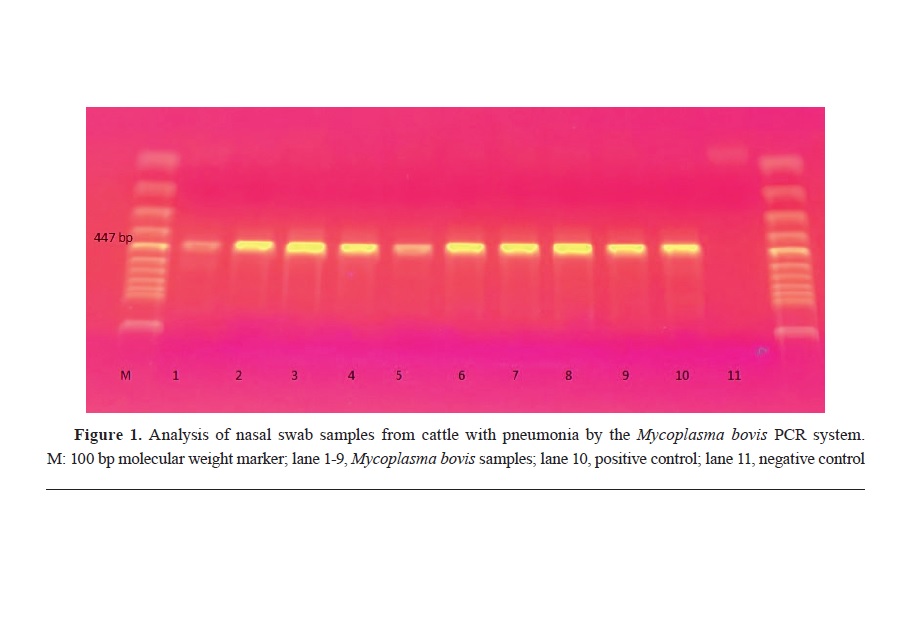
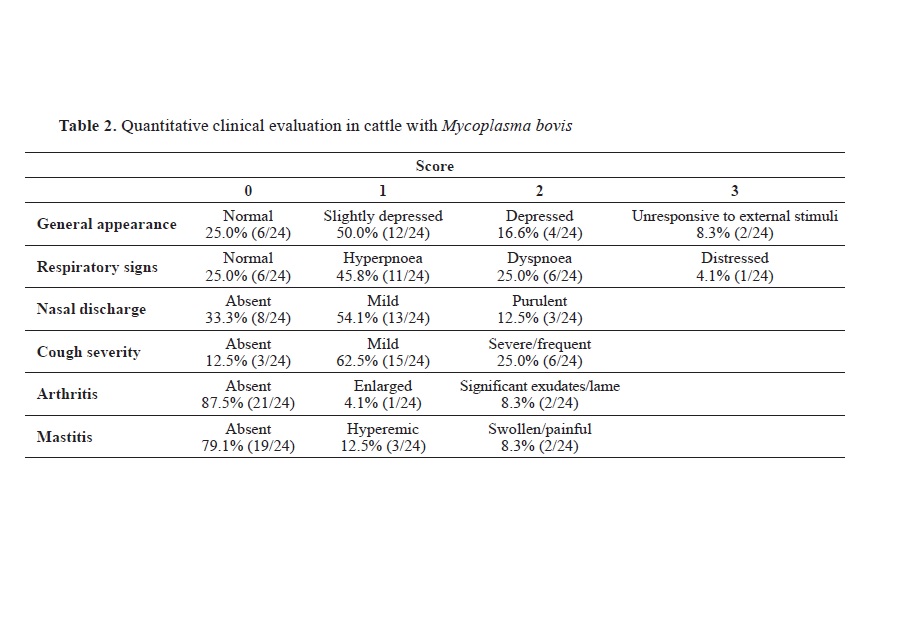
Regarding the quantitative clinical evaluation, the proportions of
M. bovis positive animals showing specific signs of disease were as follows: 50.0% mildly depressed, 45.8% hyperpnea, 54.1% mild nasal discharge and 62.5% mild cough. Arthritis and mastitis were found to have a serious course in 8.3% of animals (
Table 2).
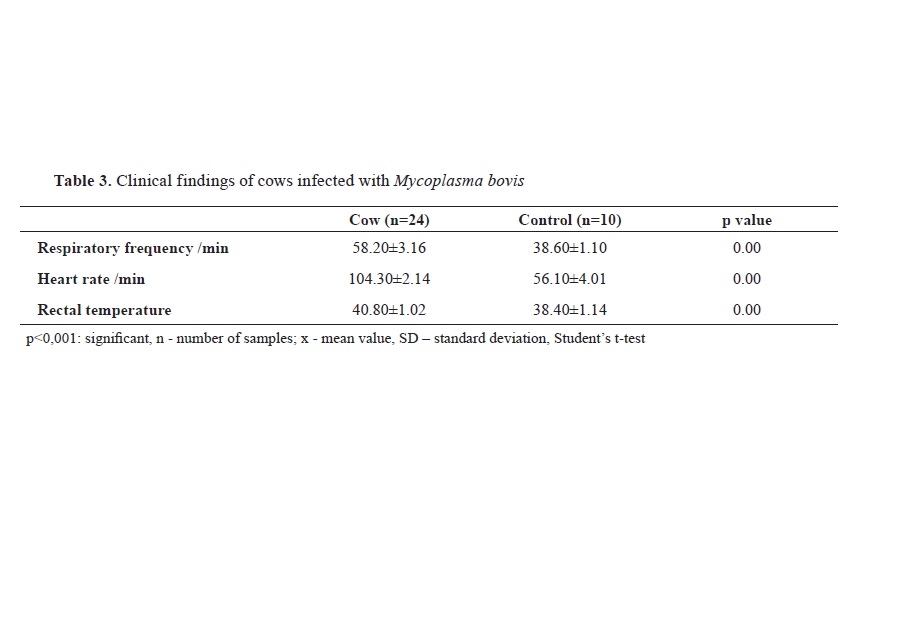
Regarding the clinical examination, respiratory frequency, heart rate and rectal temperature were higher (p<0.001) in infected animals than the control group (
Table 3).
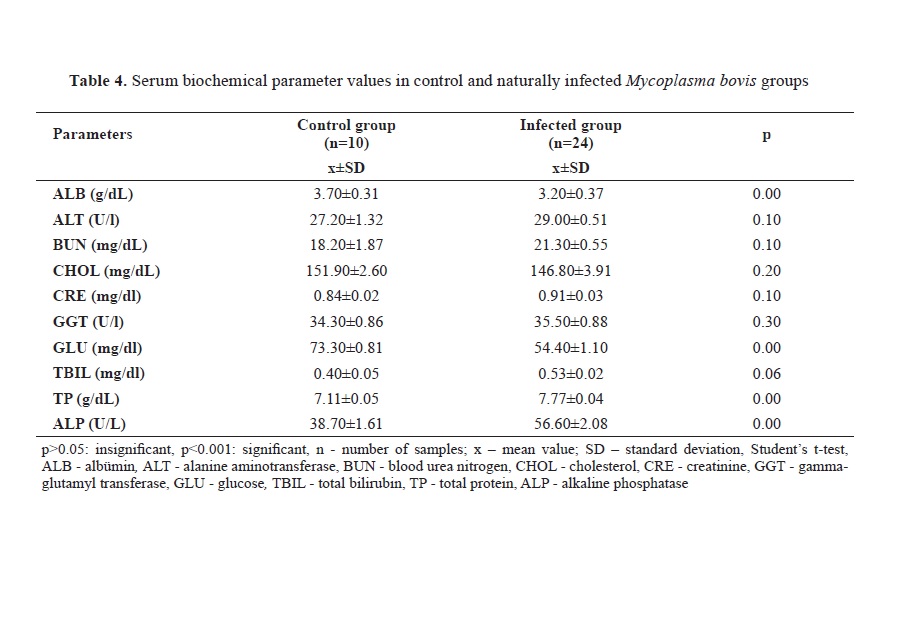
Regarding the biochemical findings, ALB and GLU levels were lower in infected animals (p<0.001) whereas TP and ALP levels were higher (p<0.001) (
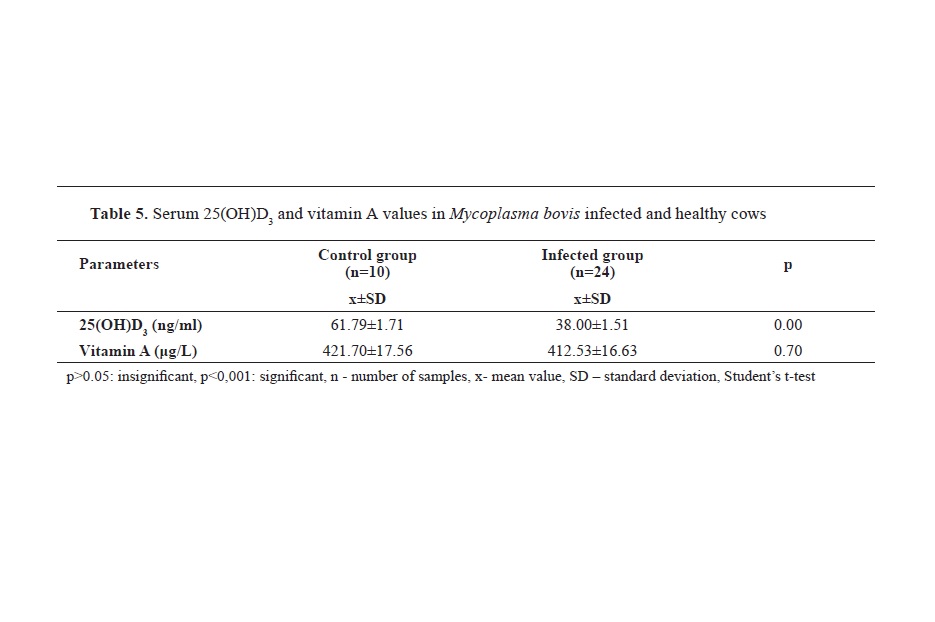
Table 4). While serum 25(OH)D
3 levels were lower (p<0.001) in infected animals, there was no significant difference in serum vitamin A levels between the two groups (p>0.05) (
Table 5).
 DISCUSSION
DISCUSSIONMorbidity and mortality rates in respiratory diseases in cattle depend on farm management, prophylactic measures, and the type of pathogen. Mycoplasma, which is an important cause of respiratory system diseases, are difficult to diagnose and control because the disease responds in varying ways to vaccination and treatment while there are also gaps in its epidemiology and pathophysiology (
13).
In this study, we used qualitative scoring to better define Mycoplasma clinical signs in cattle. This indicated that 50.0% of positive cattle had depressive appearance, 45.8% hyperpnea, 54.1% runny nose and 62.5% cough. In addition, 8.3% had serious arthritis and mastitis symptoms. Kumar et al. (
14) reported that approximately 71% of buffaloes with respiratory system disease also had nasal discharge and respiratory distress. Ramadan et al. (
15) associated shallow, rapid breathing in calves with respiratory system disease with severe inflammation, which prevents gas exchange in the alveoli.
Baruch et al. (
16) found that clinical disease score and rectal temperature correlated significantly with respiratory system diseases in cattle, while Maier et al. (
17) reported that increased respiratory rate can often be associated with respiratory system diseases. In the present study, respiratory frequency, heart rate and rectal temperature of
M. bovis positive animals were higher than in the control group. Hermeyer et al. (
18) used an experimental infection model for
M. bovis in calves. They reported higher rectal temperature and respiratory rate in chronic pneumonia cattle patients.
Regarding the biochemistry results, animals infected with
M. bovis had higher serum TP and ALP levels compared to the control group but lower ALB and GLU levels. Fraser et al. (
19) experimentally caused
M. bovis infection in calves and examined serum biochemistry values at fixed time intervals. They reported that calcium, GLU and ALP levels were higher than the reference ranges. However, they noted these values may normally be high in young animals rather than associated with pneumonia. Therefore, biochemical parameters alone may not be sufficient to define disease. They also suggested that stress may explain low serum ALP activity in calves with a high percentage of lung consolidation, decreased physical activity and increased GLU due to the anabolic state associated with pneumonia (
19). While our results are similar overall, the GLU levels were lower. This may be due to long-term malnutrition in calves with respiratory system disease (
20). Those authors also reported significantly lower serum ALB and creatinine levels in infected animals, but higher TP values. The ALB and TP values were associated with the acute phase response (
20). As the most important negative acute phase protein, serum albumin is downregulated in the acute phase response. The changes in protein profile in turn affect performance, muscle mass and carcass quality (
21). Although Šoltésová et al. (
20) reported similar results, Ramadan et al. (
15) reported higher TP, lower ALB and albumin/ globulin ratios and higher CRE levels in calves with respiratory system disease. They also suggested that the raised CRE levels could be due to impaired renal function (
15). Another research reported similar findings, that blood gas and biochemical parameters vary in respiratory system diseases (
22). Although biochemical parameters of respiratory system diseases have been frequently studied, we included them in our study because our patients had been naturally infected and had clear etiology.
In this study, serum 25(OH)D
3 levels were found to be lower in
M. bovis infected cattle. Mamani et al. (
23) found that low 25(OH)D levels were associated with higher incidence and disease severity for community-acquired pneumonia. Similarly, Kim et al. (
24) reported 25(OH)D deficiency in approximately 80% of hospitalized communityacquired pneumonia patients in South Korea. A significantly lower serum 25(OH)D levels were also reported in connection with severe pneumonia (
25). The suppressive effect of 1,25(OH)
2D
3 on the induction of inflammatory cytokines in interstitial pneumonia showed that a high vitamin D diet improved pulmonary fibrosis symptoms and reduced expression of fibrosis marker inducers (
26). Waters et al. (
27) found that 1,25(OH)
2D
3 increases
Mycobacterium bovis specific nitric oxide production, inhibits interferon gamma (IFN-γ) production and inhibits
Mycobacterium bovis specific CD4+ cell proliferation. Furthermore, low serum 25(OH)D
3 levels were associated with bovine tuberculosis, while serum levels of 25(OH)D
3> 80 ng/mL were required to counteract the infection caused by
M. bovis (
28).
In the present study, we found no significant differences in serum vitamin A levels of
M. bovis infected animals and the control group. Elnisr et al. (
29) noticed a significant decrease in serum α-tocopherol concentration in all pneumonia types in camels with pneumonia, whereas there were no significant differences in β-carotene levels. A severe pneumonia infections lead to excessive retinol loss in the urine. Thus, whereas healthy adults lose 1% of their dietary intake in their urine, intensive care patients with pneumonia and sepsis may lose around 10 μmol/day, or about three times their dietary intake (
30). Retinol levels fall particularly during the acute phase response, in correlation to the severity of the infection, before returning to normal pre-infection levels within a few days (
31). This temporary decrease may be due to the increased vascular permeability in areas of inflammation causing leakage of retinolbinding proteins into the extravascular space (
30). Thus, the lack of difference in vitamin A levels between infected and healthy animals in the present study may be because vitamin A is a fat-soluble vitamin, has a slow turnover, can be stored in the liver, and reserves may not change during the acute course of the infection (
32).
Although there are different microbiological and virological factors that cause pneumonia in cattle, as a limitation of the study, we could only examine certain microbial agents due to limited financial opportunities. However, nutrition, care, and benefiting from sunlight were similar within the group of
M. bovis and control animals studied.
CONCLUSION
In this study, it was concluded that detection of
M.bovis from samples which were obtained from animals showing respiratory tract symptoms is a potential source of risk. Due to the late response to antibiotic treatment, it is important to improve infection prevention and control strategies. Although blood and vitamin parameters are not used as biomarkers, the significant changes in these parameters in symptomatic animals suggest that measurement of these parameters can support prophylaxis, diagnosis and treatment. It is thought that more comprehensive studies will lead to the development of more effective strategies not only for mycoplasma infections but for the control and eradication of respiratory tract infections caused by other agents, as well.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThe authors would like to thank Dr. Mahmut Niyazi Mogulkoç for providing positive controls.
AUTHORS’ CONTRIBUTIONSPFPD designed the study and collected samples. ZY and BK performed PCR and microbiology analyzes. PFPD did biochemical analyzes and statistics and also wrote the article. All authors read and approved the final manuscript.

 10.2478/macvetrev-2023-0015
10.2478/macvetrev-2023-0015





