Back pain is one of the most common triggers of performance failure in athletic and riding horses. Diagnosis of equine back pain has been very challenging for equine practitioners, particularly in chronic cases. Therefore, the identification of blood biomarkers would facilitate the clinical differentiation of chronic back pain. This study aimed to investigate serum biomarkers of glial cell activation, axonal damage, and inflammation for the diagnosis of equine chronic back pain. Serum samples from forty horses comprising chronic back pain (CBP), back pain concurrent with lameness (BPL), lameness (LN), and healthy control (HC) (n=10 per group) were screened for ionized calcium-binding adaptor molecule 1 (Iba-1), glial fibrillary acidic protein (GFAP), phosphorylated neurofilament-H (pNF-H) by ELISA, and proinflammatory cytokines (IL-1β, IL-6, and TNF-α) by multiplex assay. Serum concentrations of GFAP (3.81±1.72 ng/mL) and pNF-H (0.76±0.18 ng/mL) were significantly (p<0.05) higher in horses with CBP when compared with other groups. Iba-1 was not significantly higher in CBP horses. There was no significant difference between the pro-inflammatory cytokines among the groups. The levels of IL-1β, IL-6, and TNF-α were also increased in the CBP than the HC control horses but lower in relation to BPL and LN horses. In addition, serum Iba-1, GFAP, and pNF-H showed a high discriminatory capacity for horses with CBP with high sensitivity (50-100%) and specificity (70-100%). This study provides evidence that serum levels of the GFAP and pNF-H may be useful in the clinical differentiation of horses with chronic back pain.
Equine back pain (BP) is a well-known limiting performance clinical dysfunction of the musculoskeletal structures in sport horses (
1). The etiopathological diagnosis of this condition is often difficult (
2). Currently, а definitive diagnosis of equine BP is made after eliminating other clinical conditions that may appear. These include back pain diagnosed by clinical examination, thoracolumbar radiology, ultrasonography, or response to local analgesia and anti-inflammatory drugs in cases of muscular pain in the back. However, these diagnostic approaches for BP are acceptable for horses with acute pain but not those with chronic back pain (CBP) (
3). Therefore, it is imperative to have a more objective method for the clinical differentiation of equine CBP.
Although, pain is the nervous system signal in response to a noxious insult to tissues, the development and maintenance of chronic pain has been associated with glial cell activation. Whilst the activation of glial cells serves to protect the central nervous system (CNS) from pathological changes and restore homeostasis (
4, 5), the interrelationship between the activities of neuronal and glial cells, particularly the microglia and astrocytes in the spinal cord, induces transformation of acute nociceptive pain to persistent or chronic pain (
6). This transformation occurs through the release of gliotransmitters, pro-inflammatory mediators, and lysosomal molecules (
6), resulting in neuronal hyperactivity and persistency to pain (
7).
Recently, the expression of microglia and astrocytes activation in the spinal cord dorsal horns have been demonstrated in horses with BP using glial surface markers ionized calcium-binding adaptor molecule 1 (Iba-1), and
glial fibrillary acidic protein (GFAP) respectively (
8). These markers have been used in chronic pain studies including musculoskeletal pain as indicators of microglia and astrocyte activation (
9). Phosphorylated neurofilament H (pNF-H), a biomarker of neuronal and axonal damage (
10), is used as an indicator of neuronal interactions in the development of chronic pain. Therefore, we believe that since Iba-1, GFAP, and pNF-H are soluble proteins and have been shown to be detectable in biological fluids such as blood (
11, 12, 13), their detection in blood could complement the clinical differentiation of CBP. Currently, the role of serum Iba-1, GFAP, pNF-H, and proinflammatory cytokines in equine BP and other pain-related conditions is unknown. Thus, this study investigated the diagnostic potential of the serum Iba-1, GFAP, pNF-H, and proinflammatory cytokines concentration in horses with CBP.
MATERIAL AND METHODSAnimal study and inclusion criteriaHorses presented to the University Veterinary Hospital (UVH), University Putra Malaysia (UPM) diagnosed with CBP, back pain concurrent with lameness (BPL), lameness alone (LN), and healthy control (HC) were used in the study. The horses were of various breeds namely Thoroughbred (9/40), Warmblood (5/40), Arabian (17/40), and polo pony (9/40), comprised of gelding (18/40) and mares (22/40). They were aged 5 to 22 years with a mean age of 14.40±5.13 for CBP, 14.50±3.57 for BPL, 14.50±3.44 for LN, and 11.00±3.68 for HC horses. All clinical diagnostic procedures (i.e., clinical examination, diagnostic analgesia, and thoracolumbar radiology) were done by an equine veterinarian of the VTH-UPM who is not a researcher and is not familiar with the study group’s categorization. In addition, a complete physical examination was conducted for signs of BP by experienced equine veterinarian. The evaluation of lameness, avoidance behaviors, gait abnormality, and resistance to walking was done in hand, on the lounge, and when ridden. The presence of back pain was determined by palpation along the back region and vertebral flexibility test, while the involvement of the kissing spine (impingement of dorsal spinal processes) causing the back pain was determined by radiographic examination of the thoracolumbar region. Manual palpation of the bony and soft tissues of the back region was performed with ventroflexion (sinking) and dorsiflexion (arching) of the back seen as a pain response to thoracolumbar and sacroiliac region palpation, respectively.
Therefore, based on the history and clinical examination findings, horses with persistent BP of ≥2 months, unresponsive to controlled exercise, local analgesia, anti-inflammatory, or other types of drugs, were categorized as CBP cases. The LN group were horses that exhibited lameness of grades ≥2 based on the AAEP lameness grading system (
14) while horses having lameness concurrent with back pain were grouped as BPL. The HC group were horses that showed no detectable musculoskeletal pain and were free of other clinical conditions. In total, forty horses, ten in each group (CBP, BPL, LN, and HC), were sampled. All horses included in the study had not received anti-inflammatory drugs within the last 72 h prior to sampling, however, they all had a history of vaccination against equine influenza, Japanese encephalitis, and tetanus. The study was conducted with the owner’s consent and approved by Institutional Animal Care and Use Committee, UPM (UPM/IACUC/AUP-R016/2018).
Serum sample collectionTen milliliters of blood were collected by jugular venepuncture using an 18G needle from each horse into labeled sterile plain vacutainer tubes and was allowed to clot. Clotted blood was then centrifuged at 300×g for 10 minutes, within 2 h of sample collection. Sera were harvested and stored in 1.5 mL aliquots at –80 °C.
Serum biomarkers expression studiesThe serum concentrations of Iba-1, GFAP, and pNF-H were determined via immunoassay based on the reaction of target proteinspecific peroxidase-conjugated antibody with 3,3’,5,5’-tetramethylbenzidine (TMB) as the substrate. The GFAP and pNF-H concentrations were quantified using human GFAP and pNF-H sandwich ELISA kits (EMD Millipore Corp, USA), which have been validated for use on horse serum (
12, 15). Iba-1 concentration was determined with the AIF 1 ELISA kit (Cloud-clone corporation, USA). The absorbance(s) was determined using the TECAN infinite M200PRO multi-detection microplate reader at 450 nm for GFAP and Iba-1 and 405 nm for pNF-H. The concentration of target proteins in the samples was estimated from the standard curve. All assays were done in accordance with the manufacturer’s instructions and in duplicate.
The serum concentrations of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)α were determined using the Equine cytokine/chemokine magnetic bead multiplex panel (EMD Millipore Corp, USA). The results were obtained with the use of the Luminex xPONENT software® with MAGPIX. The assay was performed in accordance with the manufacturer’s instructions and in duplicate.
Serum creatine kinase (CK) and aspartate aminotransferase (AST) were determined using the automated biochemical analyzer BiOLiS24i Premium, Version 2.0.1 (Tokyo Boeki Medisys Inc.) to rule out the possibility of myopathy causing muscular pain which could be the cause of signs, like stiffness, reluctance to move and poor performance, mimicking back pain.
Data analysisAll data were expressed as mean ± standard error of mean (SEM) except where otherwise indicated. Statistical analyses (GraphPad Prism version 8.0.2) were conducted using the one-way ANOVA followed by post-hoc Tukey’s multiple comparison tests to determine differences between means. The receiver operating characteristic (ROC) curves analysis was performed to compare the potential of the serum biomarkers in the diagnosis of equine CBP. The probability level p<0.05 was considered significant. The interpretation of ROC curve analysis was based on the area under the receiver operating characteristic curve (AUC) values. The test was scored as perfect at AUC=1, excellent at AUC=0.90-0.99, good at AUC=0.70-0.89, poor at AUC=0.60-0.69, and no discriminatory capacity at AUC=0.50-0.59 (
16). A multiple regression analysis was performed to predict CBP from the serum GFAP, Iba-1, and pNFH concentration in other to build a predictive equation for the classification of new cases.
The Coefficient model equation for the prediction of CBP given the concentration of the biomarkers was as follows: P(CBP)=0.126+0.035(GFAP)+0.02(Iba-1)+0.422(pNFH).
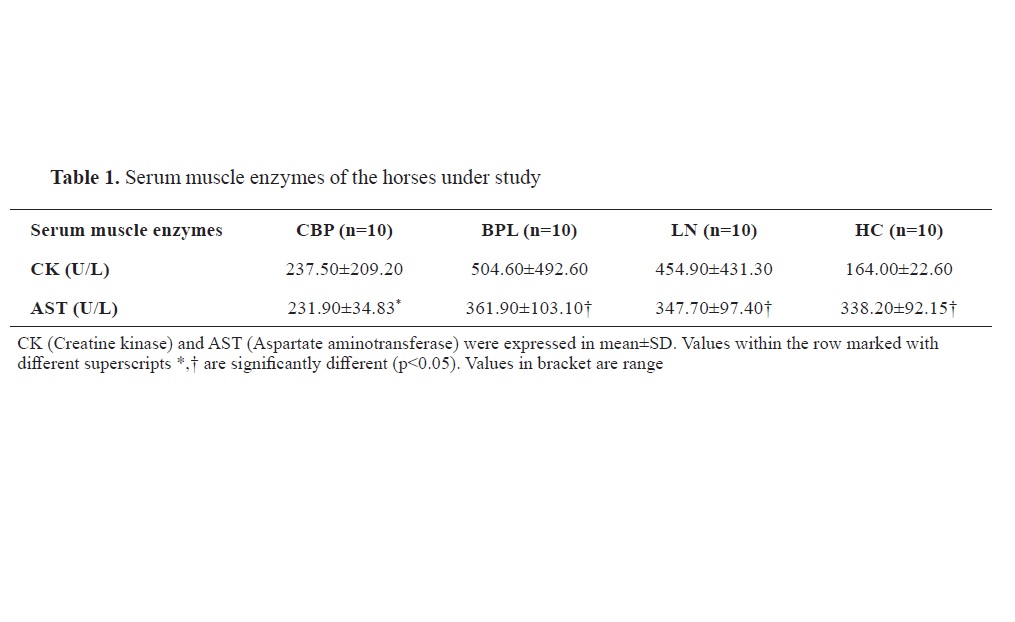
RESULTSAnimal clinical characteristicsThe serum chemistry for muscle damage is shown in
Table 1. There was no significant difference between the serum CK level among all the groups. Although AST was within the normal reference range for all groups, it was significantly lower in CBP horses when compared with the BPL (p=0.0094), LN (p=0.0241), and HC (p=0.0436) horses.
Serum biomarkersThe inter-assay coefficients of variation (CV) for GFAP, Iba-1, and pNFH in the serum samples were 10.3%, 13.5%, and 7.9% respectively. The intra-assay CV for GFAP, Iba-1, and pNFH was 5.8%, 7.3%, and 4.0% respectively. There was an increase in serum concentration of Iba-1, GFAP, and pNF-H in CBP horses compared to the BPL, LN and HC horses. The serum GFAP concentration was significantly higher (p<0.05) in CBP horses. pNF-H was higher in CBP and BPL compared to the HC horses (
Table 2). There was high variability in concentrations of serum cytokines in the horses.
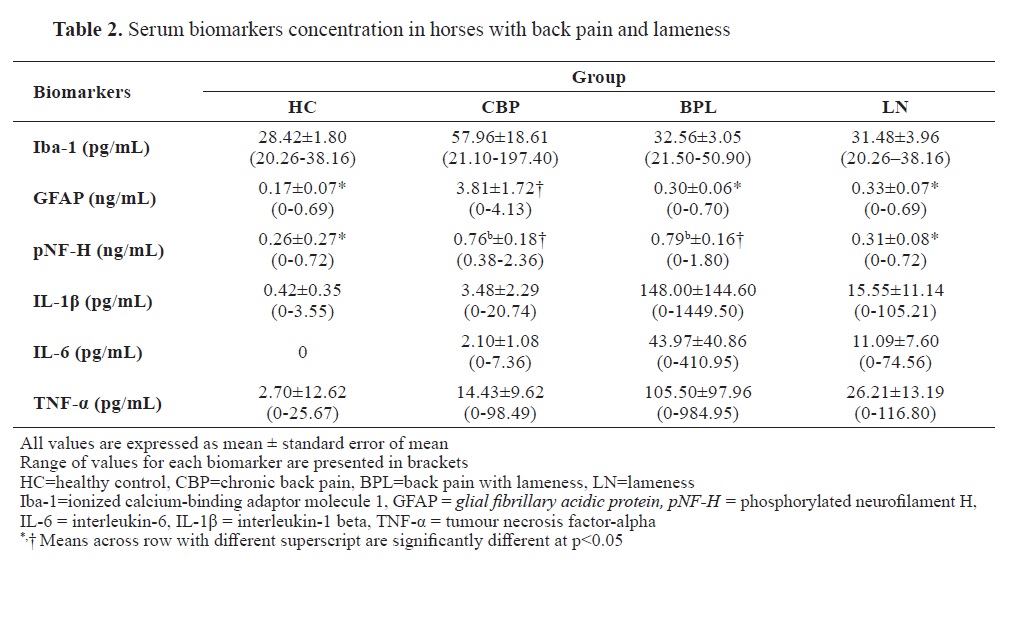
Although the concentration of IL-1β, IL-6, and TNF-α in CBP horses was higher in comparison with the HC control horses, these concentrations were however less in relation to those of BPL and LN horses. All HC horses show no detectable level of IL-6 cytokines in their serum. Similarly, IL-1β, IL-6, and TNF-α were too low to be detected in a horse from the CBP, BPL, and LN groups, respectively. Nevertheless, there was no significant difference (p>0.05) in the concentration of serum cytokines among all groups.
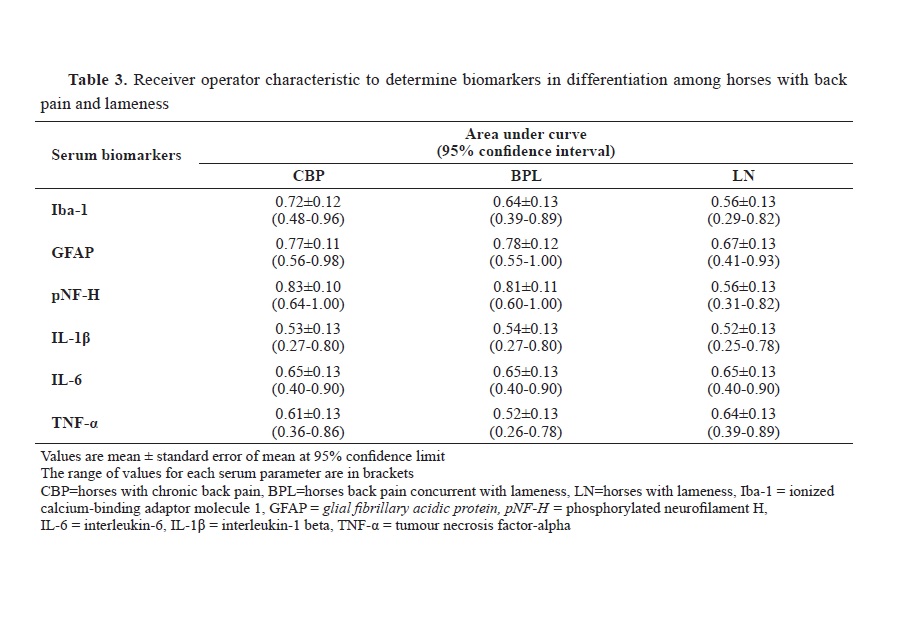
Serum GFAP at a cut-off value of 0.69 ng/mL with 50% sensitivity, 100% specificity, and AUC of 0.77 significantly differentiate horses with CBP from healthy ones (
Table 2;
Fig. 1). Similarly, horses with CBP were differentiated with serum pNF-H at a cut-off value of 0.262 ng/mL with 100% sensitivity, 70% specificity, and AUC of 0.83 (95% Cl=0.64-1.00; p=0.013). Furthermore, the cut-off value for Iba-1 to differentiate CBP from healthy horses was 26.99 pg/mL with 80% sensitivity and 70% specificity. Using the three biomarkers: GFAP, Iba-1, and pNFH, multiple regression was run to predict CBP in horses. The regression model shows a good fit for the data with the three biomarkers statistically significantly predicted CBP (F
(3, 16)=3.513, p=0.040, R2=0.397).
The cytokines IL1β, IL-6, and TNF-α (
Table 3) showed poor or no discriminatory capacity and cannot be used to differentiate between horses with and without back pain or between those with CBP, BPL, and LN.
DISCUSSIONThe clinical signs for BP in horses are nonspecific and pathological features vary with cases. Though diagnostic imaging techniques like radiology, ultrasonography, and scintigraphy have improved clinical diagnosis of either vertebral or soft-tissue structures causing back pain in horses, clear radiographic changes of articular process joints can be difficult to visualize due to the superimposition of bone structures. Currently, the only objective method of quantifying musculoskeletal back pain through the determination of mechanical nociceptive threshold (MNT) values is pressure algometry (
17). However, its result could be influenced by certain confounders such as time of the day, differences in pain sensation (or tolerance) among horses, rate, duration of the pressure applied, and avoidance responses of horses due to previous experiences (
18, 19). Therefore, the use of biomarkers to differentiate CBP from other pain-related musculoskeletal disorders such as myopathy and lameness could complement the objective method of back pain diagnosis. Muscle disease can easily be determined by estimating concentrations of serum muscle enzymes, CK, and AST. Incidentally, in our study, horses with or without BP showed normal muscle enzyme concentrations, suggesting that the CBP was not due to abnormal muscle conditions or disease.
We investigated the potential of proteins in serum that originate from the glial cell and axon, and serum proinflammatory cytokines as biomarkers for the diagnosis of CPB in horses. The subjects also included horses with BPL, and with lameness only. Iba-1 and GFAP are known to be serum biomarkers for pain and sensation as a result of microglia and astrocytes activation, respectively (
20, 21, 22). They can be easily analyzed in blood serum (
11, 16, 23) and can be used by clinicians for disease diagnosis and monitoring in horses.
As anticipated, the results showed that BP sensitization due to microglia and astrocyte activation in these horses can be determined by the increase in serum Iba-1 and GFAP concentrations, respectively. Horses with CBP showed higher serum Iba-1 and GFAP levels than those with BPL, LN, or HC horses. Serum GFAP concentrations were particularly highest in horses with CBP among groups. Horses with BPL showed low serum GFAP concentrations with values in the range similar to that of HC and LN horses. Thus, if GFAP is presumed to be an indicator of CBP-associated astrogliosis (
6, 21, 22, 24), then the clinical signs of pain in horses with BPL in this study may be a manifestation of lameness rather than BP from spinal disorder precipitated by astrogliosis. It has been documented in humans with chronic pain that the CSF GFAP concentrations are elevated (
20). It was also reported in dogs that serum GFAP can be used as prognostic or diagnostic markers for intervertebral disc disorder causing spinal cord injury (
13, 25). Thus, this is evidence suggesting that GFAP can be used as a biomarker for spinal injury related equine BP.
The development of chronic pain is associated with neuronal and glial activities (
26) and the release of their markers following activation or during damage (
27). Furthermore, pNF-H has been identified to be a diagnostic marker for neurological disorders in horses (
12, 15, 28). Interestingly, in our study, the serum pNF-H concentrations were significantly higher in horses with CBP and BPL than those with LN or HC. It is suggested that there is some degree of axonal loss associated with CBP in horses (
8).
The serum pro-inflammatory cytokines, IL-1β, Il-6, and TNF-α were also higher in CBP than in normal horses. Although the study did not show significant differences, BPL and LN horses showed higher serum cytokines levels than those with CBP. This finding is consistent with that of earlier reports in humans which show the association between chronic low back pain and an increase in the expression of pro-inflammatory cytokines (
29, 30). In back pain, an increase in serum cytokines is not only the result of an increase in neuronal and glial activities but also from an increase in autocrine signaling activity (
31). We believe that an increase in serum proinflammatory cytokine in BPL and LC horses may suggest high pain intensity that is associated with musculoskeletal tissue inflammation caused by lameness rather than spinal abnormality (
32). In our study, the level of serum cytokines in horses with CBP was non-significantly higher than in horses with other abnormalities, suggesting that these serum biomarkers only contribute marginally to their serum concentrations.
The ROC curve analysis suggested that the serum Iba-1, GFAP, and pNF-H are potentially better biomarkers for CBP pain in horses than proinflammatory cytokines. More importantly, serum GFAP and pNF-H concentration can be used to differentiate between horses with true BP and those manifesting secondary BP due to lameness. Based on the Youden index, the diagnostic performance of serum GFAP, pNF-H, and Iba-1 in the detection of equine CBP is 0.69, 0.262 ng/mL, and 26.99 pg/mL, respectively. The cut-off values for GFAP and Iba-1 in the differentiation of horses with various back-associated abnormalities are much lower than those reported in the dogs with spinal cord injury due to intervertebral disc injuries (
13, 24). A much higher concentration was reported in humans with poor-outcome CNS injuries. The lower concentration of these biomarkers in BP was presumably the result of neuroinflammation due to the potentiation of sensory neurons in the spinal dorsal horn following glial activation rather than direct CNS injury reported in the dog or human (
16, 33, 34). However, the study suggests that serum GFAP, Iba-1, and pNF-H are potentially good diagnostic markers for CBP in horses. To the best of our knowledge, this is the first report on the increase in serum glial cell and axonal damage biomarker concentration in CBP horses.
The result of this present study is limited due to the smaller sample size and therefore the study may lack adequate power. However, considering the findings that show the potential of biomarkers like GFAP, Iba-1, and pNF-H for the clinical differentiation of CBP. This study could serve as baseline information for future studies on biomarkers investigation in the diagnosis of CBP in horses.
CONCLUSION
Horses with CBP showed an increase in serum Iba-1, GFAP, pNF-H, and pro-inflammatory cytokines concentrations. The serum Iba-1, GFAP, and pNF-H levels reflected the glial-neuronal relationship in chronic pain and are potential biomarkers for equine CBP.
CONFLICT OF INTERESTThe authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTSThis study was supported by the Fundamental Research Grant Scheme (FRGS/1/2017/SKK15/UPM/02/2) from the Malaysian Ministry of Education. The authors are grateful to the resident veterinarians at Large Animal Clinics, University Veterinary Hospital, University Putra Malaysia (UVH-UPM) for their assistance and horse owners for consent to use their horses.
AUTHORS’ CONTRIBUTIONThe study was designed by IA and RA. AM and NA were involved in sample collection. Data curation and analysis were done by AM and MM. Fund was secured by IA. The manuscript was drafted by AM and critically reviewed and edited by IA, NA, MM and RA. All authors gave their final approval of the manuscript.

 10.2478/macvetrev-2023-0016
10.2478/macvetrev-2023-0016



