There is a lack of data about
Mhyo infection and associated lung lesions in Macedonian pig farms. The objective of this study was to determine
Mhyo in five Macedonian commercial pig farms with history of respiratory diseases by using serology testing and lung lesion scoring at slaughter.
MATERIAL AND METHODSThe current study was conducted in five commercial farrow-to-finish pig farms (A, B, C, D, and E) presented a history of clinical respiratory diseases:
Farm A had 80 sows in herd and reported clinical respiratory disease associated with enzootic pneumonia in pigs at the age of around 16 weeks. The farm was seropositive to porcine respiratory and reproductive virus (PRRSV) and no medication protocols were being used. Pigs were vaccinated at the age of around 28 days using single bivalent vaccine Suvaxin, Circo+MH RTU (Zoetis, Belgium SA).
Farm B was a 150-sows herd and severe respiratory clinical signs were recorded in nursery pigs at 10 weeks of age. The farm was positive for PRRS according to PCR and ELISA. Vaccination against PRRSV was not performed. A sulfamethoxazole-trimethoprim combination (400 ppm) along with tiamulin (150 ppm) were given in feed for 10 days after weaning (prestarter feed) and repeated for 7 consecutive days when piglets were about 50 days old (starter feed). Weaned pigs at 30 days of age were vaccinated with SuvaxinCirco+MH RTU (Zoetis, Belgium SA).
Farm C had 170 sows and 8-week-old nursery pigs affected by poor growth rate, dyspnea, cyanosis, and rough hair coat (approximately 20-30% of the pigs). The farm had pigs vaccinated at the age of 21 days for
Mhyo with one dose of 2 ml M + Pac (Schering Plough, Animal Health), while vaccination against porcine circovirus 2 (PCV2) was performed at weaning at the age of around 28 days with 2 ml dose of Suvaxin, Circo (Zoetis, Belgium SA). The farm was PRRS positive and no vaccination was performed. Medication protocols were not being implemented.
Farm D was a 170-sow herd and severe respiratory signs were observed in 9-week-old nursery pigs which continued until 14 weeks of age in grower pigs. Antibodies against PRRSV were detected in all age categories. Vaccination of weaned pigs (28 days of age) against
Mhyo and PCV2 was performed with Suvaxin, Circo+MH RTU (Zoetis, Belgium SA). Medication protocols were not used.
Farm E had 125 sows in breeding herd. Dry, nonproductive cough affecting 10 to 20% of the pigs aged 16 to 18 weeks in the finishing barns was recorded. The farm was positive to PRRSV. Pigs were vaccinated at weaning with Suvaxin, Circo+MH RTU (Zoetis, Belgium SA). Medication protocols were not implemented.
All farms included in the study had farrow-to-finish production system and all-in-all-out (AIAO) practices in nursery and finishing units were managed only by pen.
Blood samples were obtained during a single farm visit. A total of 250 blood samples (50 samples per farm) were taken randomly from five different pig categories including 6, 10, 14, 18, and 22 weeks of age. Ten animals per age group from each farm were restrained and blood sampled from the external jugular vein with a 19-gauge needle using vacuum blood tubes without anticoagulant for serum retrieval. Blood samples were placed in a cooler with icepacks and were transferred to the diagnostic laboratory.
After centrifugation of the blood samples at 300 g for 10 min, the obtained serum was used to detect antibodies against
Mhyo. Serological examination was carried out using Enzyme Linked Immunosorbent Assay–ELISA with the commercial kits IDEXX (Westbrook, ME, USA) M. hyo Ab Test according to the manufacturer’s recommendations. The level of antibody in the sera was determined by calculating the sample to positive (S/P) ratio. Samples with cut-off values of ≥0.4 were considered positive.
At the slaughterhouse, 250 lungs (50 lungs per farm) from pigs slaughtered at market weight were evaluated and scored for lesions. Lungs were scored for enzootic pneumonia (EP)-like lesions using the LLS method by Madec and Kobisch (10). Each of the lobe was individually assessed and scored according to the affected surface using 0-4 point scale. Depending on the percentage of affected lobe surface, score zero was given when no lesions were detected, score 1 was for lesions affecting <25% of the lobe surface, score 2 for lesions affecting 25-49% of the surface, score 3 for lesions affecting 50-74%, and score 4 was given for lesions affecting >75% of the lobe surface. The total score of each lung ranged from 0 to maximum 28 points.
Statistical analyses were performed using STATISTICA (version 8.0; StatSoft, Inc). Chi square-test and the Fisher’s exact test were preformed in order to find the differences in the frequency of serologically positive pigs of the same age group among the farms. Descriptive statistic was applied for data obtained on the slaughterhouse, while non-parametric Kruskal-Wallis test was used to determine the significance of the lung lesions scores found among the farms. The results were considered statistically significant at p<0.05.
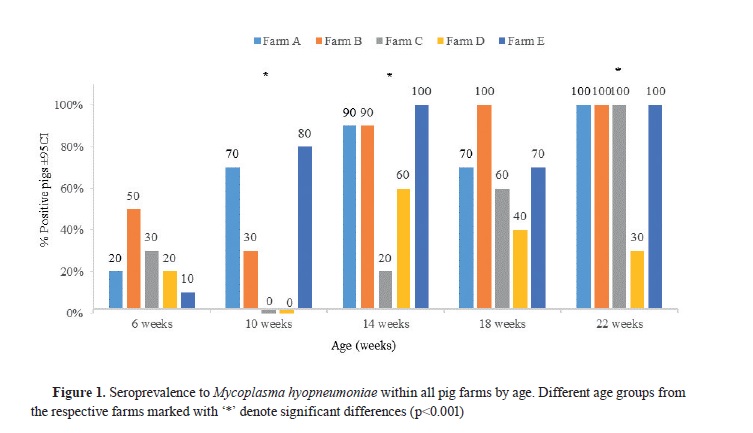
RESULTSAll of the investigated pig farms were seropositive to
Mhyo. The frequency of seropositive
Mhyo in the different age groups in each farm is presented in
Fig. 1. Pigs at the age of 6 and 10 weeks had lower seroprevalence to
Mhyo compared to the other age groups. At 10-, 14-, and 22-week-old pigs, statistical differences (p<0.001) were observed among different farms (
Fig. 1). Additionally, farms A and E showed similar serological trend, while 10-week-old pigs in farms C and D were seronegative to
Mhyo. Lower percentage of positive animals in farms C and D was observed in the 14-week-old group compared to the same age group from the other farms. High proportion of
Mhyo seropositive pigs at the age of 22 weeks were found in all farms except in farm D (
Fig. 1).
The occurrence of EP-like lung lesions was found in 91.2% of the samples ranging from 82 to 98% per farm. The lesion score inside farms ranged from 0 to 27, while mean score for all samples was 11.50. Highest mean LLS was found in Farm D (14.4), while the lowest score was observed in farm C (8.04). Descriptive data regarding LLS for all farms are shown in
Table 1.


The highest samples with typical EP lesions (60.4%) had LLS ≥10 compared to the samples with scores 1-5 (12.4%) and 6-10 (18.4%). The distribution of samples with LLS score ≥10 in farm C was significantly lower (p<0.001) than farms A and D (
Table 2). Significantly differences among farms for other LLS grades were not detected.
The medians of LLS found in farms C and B were significantly lower (p<0.001) than medians obtained in farms A and D (
Fig. 2).
 DISCUSSION
DISCUSSIONIn the current study, five Macedonian pig farms reporting clinical signs of pneumonia were evaluated for
Mhyo infection by serology testing and lung lesion assessment. To the best of the authors knowledge, this was the first study assessing the clinical
Mhyo infection in Macedonian commercial pig farms.
According to our results for serology of
Mhyo, seropositivity detected in farm A and E in 10-week-old pigs was most likely due to vaccination. In addition, high percentage of seropositive pigs at the age of 22 weeks (slaughter weight) was observed in farms A, B, C, and E which is in compliance with the findings of other authors. Galdeano et al. (
11) found that more than 90% of the finishing pigs in 23 out of 29 vaccinated pig herds were seropositive to
Mhyo. Similarly, in the study conducted by Tassis et al. (
12),
Mhyo antibodies have been detected in 100 % of vaccinated pigs at slaughter age. High serological response in the currents study observed in finishing pigs is most likely associated with the immune-boosting effect of vaccination which stimulates significant antibody production due to field infection (
12). Thus, positive immune response of
Mhyo vaccination and antibody production in pigs against natural infection was previously documented (
12, 13). On the other hand, low percentage of seropositive pigs that we observed in farm D is in line with the results reported by Sibila et al. (
8), where in some herds percentage of vaccinated pigs seroconverted to
Mhyo at finishing stage was up to 10%. This low proportion of seropositive pigs in farm D could be a result of inadequate administration, storage or timing of vaccines. Nevertheless, seroconversion to
Mhyo is variable in pigs (
6) since antibodies are detectable approximately 3 to 8 weeks post exposure in both infected (
2) and vaccinated animals (
11). However, we could not differ vaccine antibodies from those produced by infection, which certainly limits interpretation of the data.
Moreover, ELISA results should be taken with caution since infections with other nonpathogenic mycoplasmas, like
Mycoplasma flocculare, could be the cause of unexpected false‐positive rates (
14). Serology sample size in this study could be insufficient for detection of diseases prevalence, and hence dynamics of
Mhyo infection was roughly estimated. However, the number of samples per pig category for serum profiling in the current study is similar with other studies
(6, 15).
High percentage of lungs with EP-like lesions (91.2%) obtained in our study was close to the results reported by other researchers (
11, 12). Galdeano et al. (
11) in their survey found that 80.3% of the examined lungs had EP lesions, while Tassis et al. (
12) identified that 94% of the lungs from vaccinated pigs at slaughter weight were associated with typical EP lesions. However, the high percentage (91.2%) of lungs with EP-like lesions does not correspond with data obtained by some other researchers (
2, 4, 16, 17) who found lower percentage (46.4 – 59.6%) of pig lungs affected by EP. Although, vaccination against Mhyo was performed in all farms, we found huge differences among LLS in the farms. The highest mean LLS and the lowest percentage of seropositive 22-week-old pigs that was found in farm D is in line with the results reported by other studies. Andreasen et al. (
18) found that pigs close to slaughter age, seroconverting or not seroconverting for
M. hyopneumoniae, had the highest degree of lung lesions. In another study, higher percentage of seropositive pigs at 20 weeks of age had significantly better mean LLS than the group with lower percentage of pigs that were seropositive (
12). Several factors such as management, environmental conditions (
19) and strain virulence (
20) may influence on the severity of lesions. Coinfection with more than one
Mhyo strain could also be associated with severe lung lesions
(21, 22).
Nevertheless, our study was limited since it was cross–sectional, including small number of farms, and it did not reflect the real picture of
Mhyo infection in the country. Further research with larger sample sizes should be done in order to reveal more insight and information on this respiratory infection in commercial pig farms in Macedonia.
CONCLUSION
This study gives novel data about
Mhyo infection in Macedonian commercial pig farms. High proportion of finishing pigs seropositive to Mhyo was probably due to vaccination. Farms with high proportion of seropositive pigs to
Mhyo were associated with low LLS. Serological testing to
Mhyo from different age groups and slaughter check assessment of finishing pigs is an excellent approach for assessing the vaccination status of pig herds.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSPig farms are acknowledged for their contribution to this study. The authors would like to thank the colleagues from the Laboratory of Serology from the Faculty of Veterinary Medicine in Skopje for their technical support with the ELISA testing.
AUTHORS’ CONTRIBUTIONSBA developed the study design and concept, collected samples, conducted a lung assessment and wrote a manuscript. CMO revised the manuscript. AJ was involved in lung assessment and samples’ collection. AD was involved in data analysis and interpreted ELISA results. RP contributed in manuscript writing and was involved in interpretation of data. JB contributed in the analyzing data and gave critical review for important intellectual content. All authors have revised and approved the final version of the manuscript.

 10.2478/macvetrev-2023-0018
10.2478/macvetrev-2023-0018



