Brilliant cresyl blue (BCB) staining is a method used for selection of developmentally competent porcine oocytes, in addition to the standard morphological classification. The aim of the current study was to assess the relationship between BCB staining in porcine oocytes with high and low morphological characteristics and its maturation rate. In the first part of the study, cumulus-oocytes complexes (COCs) (n=434) were aspirated from 60 ovaries. According to the morphological classification and BCB staining, they were divided in 4 groups: COC+/BCB+, COC-/BCB+, COC+/BCB-, and COC-/BCB-. In the second experiment, COCs (n=445) were categorized into 3 groups: control, BCB+, and BCB-. Significant differences in the maturation rate were observed between COC+/BCB+ and COC-/BCB- (66% vs. 23%), and between COC+/BCB+ and COC-/BCB+ (66% vs. 38%) (p<0.0001). Significant differences were also registered between COC-/BCB+ and COC-/BCB- (38% vs. 23%) and between COC+/BCB- and COC-/BCB- (53% vs. 23%) (p<0.01). Results from the second experiment showed that only BCB- oocytes had a significantly lower maturation rate (28%) compared to the control (63%) and BCB+ oocytes (59%) (p<0.001). These findings showed that COCs with high morphological characteristics had higher developmental ability compared to COCs with low morphology. The BCB-staining in high-quality oocytes did not have a significant impact on their maturation ability compared to a control group, but it might be useful for selecting developmentally competent oocytes with low morphology characteristics.
Incomplete nuclear and ooplasmic maturation of the oocytes has an impact on unsuccessful fertilization. Oocytes used for
in vitro production are most frequently retrieved from ovaries obtained from slaughterhouses. It is highly important for these oocytes to be assessed prior to their use in IVP because they can be in different developmental stages (
5). The primary criterion widely used for oocyte quality assessment is morphological classification. It is based on the number of layers and compactness of cumulus cells surrounding the oocyte, as well as the homogeneity of the ooplasm (
6). However, some studies in cattle and other species show that the morphological classification is insufficient because up to 60% of the IVM/IVF oocytes fail to reach the blastocyst stage (
4). It is believed that one of the causes is an early stage of degeneration of oocytes with normal morphology (
7). Currently, there is no reliable method for measurement of ooplasmic maturation (
3).
In the 90s’, Ericsson et al. (
8) suggested a simple method for selection of porcine oocytes based on the enzymatic activity of glucose-6-phosphate dehydrogenase (G6PDH), a component of the pentose cycle synthesized during the oogenesis. This enzyme is responsible for the production of ribose for synthesis of nucleotides, as well as NADPH for lipid synthesis during early development (
8). It has been established that G6PDH gets inactivated as soon as the oocytes complete their growth (
4). The catalytic activity of G6PDH in immature oocytes on brilliant cresyl blue (BCB) renders it colorless (
9). Oocytes with completed growth phase have blue colored ooplasm and are classified as BCB-positive (BCB+). Due to the high enzymatic activity of G6PDH in growing oocytes, the BCB stain is metabolized, the oocytes remain unstained, and are categorized as BCB-negative (BCB-) (
10).
Due to the non-invasiveness and possibility for further manipulation of the oocytes, some authors consider that the BCB staining method should be verified as an indirect indicator of the quality and developmental competence of the oocytes. Others, however, are against it, based on the findings that, in some animal species, the blastocyst rate of BCB+ oocytes was not significantly higher compared to the control group (
3).
There are scarce data in the literature about the maturation rate of porcine oocytes that have been classified as BCB-. However, so far, we did not find any studies that have used the BCB staining method for oocytes that are classified as low quality, since they are usually excluded from further analysis.
Based on the above, we hypothesized that besides the morphological assessment, the BCB staining method would additionally help to elucidate the quality of the immature oocytes before their usage in IVP. Therefore, this study aimed to assess the correlation between BCB-positive oocytes with high and low morphological characteristics and their maturation rate compared to a non-treated (control) group.
MATERIAL AND METHODSOvaries’ collection and aspiration of cumulusoocyte complexesPorcine ovaries (n=119) were obtained from prepubertal gilts from two local abattoirs and transported to the laboratory in a thermos (Minitub GmbH, Germany) containing sterile solution of 0.9% NaCl with gentamicin (50 μg/mL) (
11) at 38 °С within 2 h after animals’ slaughter.
Upon arrival in the laboratory, ovaries were washed 3 times with warmed sterile saline (38.8 °С). The cumulus-oocyte complexes (COCs) were aspirated from follicles (3-6 mm) using 20-G needle connected to vacuum pump (COOK IVF, William A Cook Australia Pty Ltd, Australia) with vacuum pressure of 60 mmHg (
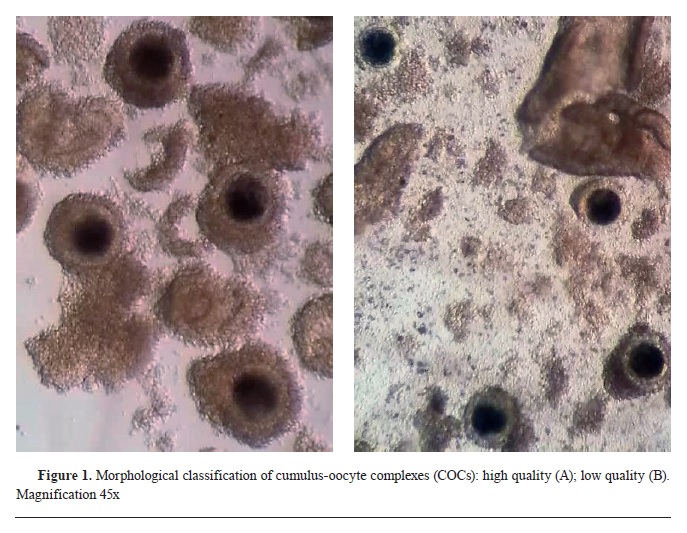
12). The COCs were then transferred in washing medium (WASH, IVF Bioscience, UK) and morphologically classified under a stereomicroscope (Hund Wetzlar, Minitub GmbH, Germany) with 45x magnification into two experimental groups: 1) COC+ - high quality (compact cumulus oophorus, ≥3 layers of cumulus cells, homologous ooplasm) and 2) COC- - low quality (incomplete cumulus oophorus, ≤3 layers of cumulus cells, fragmented ooplasm) (
Fig. 1).
Brilliant cresyl blue stainingPrior to staining, the COCs were washed 3 times in mDPBS (Dulbecco’s phosphate-buffered saline, Gibco, Life Technologies Limited, UK, supplemented with 0.4% bovine serum albumin-BSA, Merck, Darmstadt, Germany), then transferred in a 4-well dish (Thermo Fisher Scientific, Roskilde, Denmark) containing mDPBS with 26 μM BCB (Sigma-Aldrich Co, St. Louis, MO, USA) (
13). They were incubated for 90 min in a humified air atmosphere at 38.8 °C and 6% CО
2, after which the rate of BCB staining was assessed.
Experimental designTwo experiments were conducted during this study.
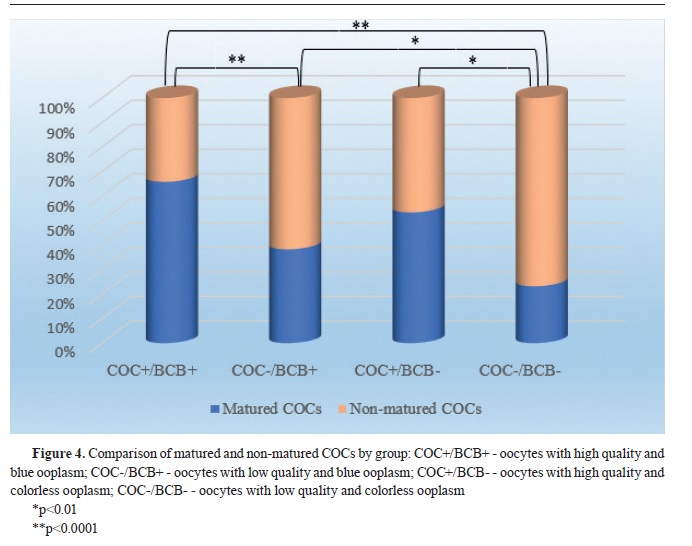
In the first experiment, after morphological classification, both COC+ and COC- (n=434) were exposed to BCB staining. After the incubation, COCs were washed twice in mDPBS and classified in 4 experimental groups using stereomicroscope: 1) COC+/BCB+ - oocytes with high quality and blue ooplasm; 2) COC-/BCB+ - oocytes with low quality and blue ooplasm; 3) COC+/BCB- - oocytes with high quality and colorless ooplasm; 4) COC-/BCB-- oocytes with low quality and colorless ooplasm. The oocytes from all groups were subjected to maturation.
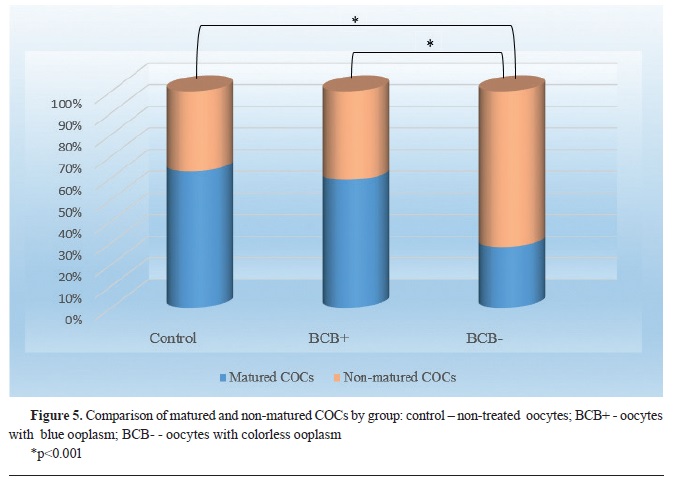
In the second experiment, only COCs with high quality were selected for further manipulation (n=445). Eighty (n=80) COCs were immediately transferred into maturation medium (control group). The remaining COCs were stained with BCB and divided into 2 groups: 1) BCB+ - blue ooplasm; and BCB- - colorless ooplasm. The oocytes from all groups were subjected to maturation.
 In vitro maturation
In vitro maturationThe COCs from each group in the first experiment were washed in maturation medium (BO-IVM™, IVF Bioscience, UK), transferred to 4-well dish and maturated
in vitro for 40-42 h in a humified air atmosphere at 38.8 °C and 6% CО
2. After this period, the maturation rate in each group was determined, based on the expansion of the cumulus cells.
The same procedure has been repeated in the second experiment, except that the duration of the
in vitro maturation of the oocytes lasted 44 hours.
Statistical analysisNominal variables were expressed as absolute numbers (contingency tables 2x2, 3x2, and 4x2) and analyzed with Pearson’s Chi-squared test’ using the ‘R version 4.1.3, 2022’ software. Post-hoc analysis was performed with Pairwise test of association. The statistical power was tested with G*Power Version 3.1.9.2 Software based on the alpha value 0.01, total number of samples and degree of freedom 1, 2, and 3. The values are presented in percentages of the total number of oocytes in corresponding groups.
RESULTSFirst experimentA total number of 147 oocytes with high quality (COC+) and 287 oocytes with low quality (COC-) were exposed to BCB staining in the first experiment (
Fig. 2).
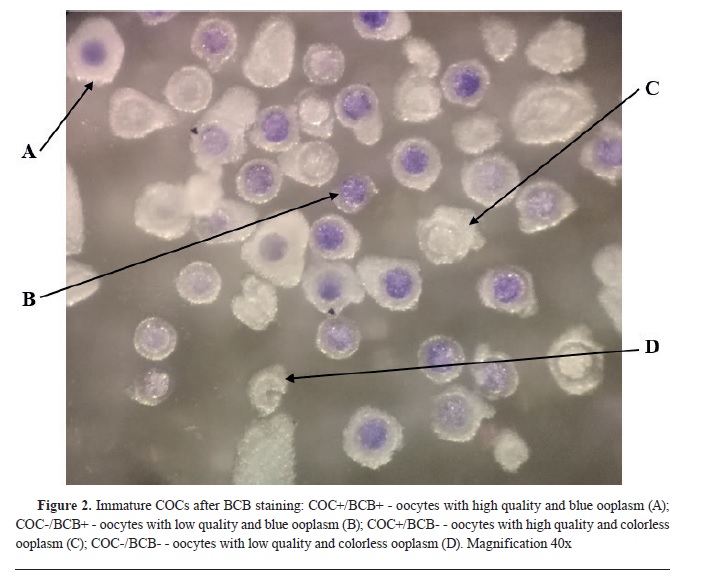
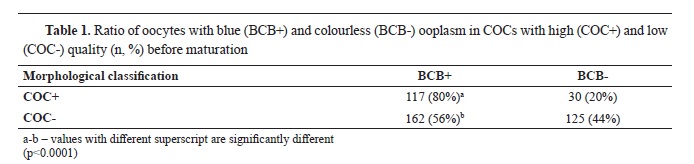
The ratio of oocytes with blue (BCB+) and colorless (BCB-) ooplasm in high quality and low quality oocytes was 80% vs. 20%, and 56% vs 44%, respectively (
Table 1). There was a significantly higher number of oocytes with high quality that were classified as BCB+ (χ2=41.18, p<0.0001).
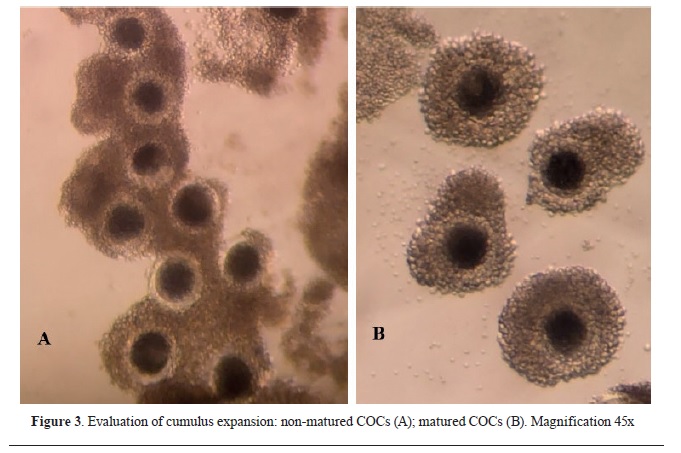
After the 40-42 h incubation, an assessment of the maturation rate of the oocytes was made. COCs with expanded and loose cumulus cells were considered as matured (
Fig. 3).
Statistical analysis of the obtained results has shown that the most significant difference (p<0.0001) of the maturation rate was found in COC+/BCB+ (n=77; 66%) compared to COC-/BCB- (n=29; 23%), as well as in COC+/BCB+ compared to COC-/BCB+ (n=77; 66% vs. n=62; 38%, respectively). There was also significant difference (p<0.01) between COC-/BCB+ (n=62; 38%) and COC-/BCB- (n=29; 23%), as well as between COC+/BCB- and COC-/BCB- (n=16; 53% vs. n=29; 23%). The maturation rate between COC+/BCB+ and COC+/BCB− (n=77; 66% vs. n=16; 53%) as well as between COC+/BCB and COC-/BCB+ (n=16; 53% vs. n=62; 38%) was not significantly different (
Fig. 4). The value of Pearson’s Chi-squared test was χ
2=47.72.
 Second experiment
Second experimentThe results in the second study with high quality oocytes showed that only the BCB- group had significantly lower (p<0.001) maturation rate (n=55; 28%) compared to the control group (n=50; 63%), as well as the BCB+ (n=98; 59%). The difference of the maturation rate between BCB+ and control group was not statistically significant (
Fig. 5). The value of Pearson’s Chi-squared test was χ
2=46.08.
DISCUSSIONThe selection of porcine oocyte with developmental competence might be enhanced with combining more classification methods. In the first experiment of this study, our results showed that 38% of the oocytes with low morphological characteristics and stained ooplasm (BCB+) matured
in vitro and could be used for further analysis. Furthermore, it was found that regardless of the presence or absence of BCB-staining, COCs with high morphological characteristics always expressed significantly higher developmental ability evidenced by higher maturation rate compared to COCs with low morphological characteristics. Those results corroborate with reports that most laboratories use IVF protocols based on COCs’ morphology (
14).
The justification for using BCB staining as a routine method in
in vitro embryo production still remains an open question, having its supporters and opponents (
3, 14). Some studies showed no significant differences in the developmental potential of porcine and equine embryos produced by either the BCB+ oocytes or the control group (
5, 14). The absence of a significant difference in the maturation rate between high quality oocytes in the control and BCB+ group in the second part of our study, leads to an assumption that the oocytes from these groups had similar developmental competence, and consequently, might not have significant differences in the embryo developmental potentials. The insignificant difference registered between the maturation rate of control (63%) and BCB+ (59%) oocytes in this study corresponded with the results of Pawlak et al. (
14) (91.4% vs. 88.8% p>0.05, respectively), who found high similarity in parameters connected to cytoplasmic maturation of BCB+ and control oocytes. Also, reports from Opiela et al. (
4), showed no significant difference of the blastocyst rate between control and BCB+ oocytes.
However, our findings are not consistent with data from Ericsson et al. (
8) who compared the maturation rate of oocytes with different color intensities (dark-retained color, intermediatepartial color, and clear-no color) with the control group. Those authors found that the maturation rate of the dark oocytes was significantly higher (82%, p<0.05) compared to the control group (65%). The differences between our results might be due to the different concentrations used for BCB staining since in our experiment oocytes were incubated in 26 μM BCB, while in the study of Ericsson et al. (
8) the concentration of the stain used for oocyte incubation was 13 μM BCB. Also, a different classification methodology of the stained oocytes was used in the study.
The research of Ishizaki et al. (
5) reported statistically higher meiotic competence in porcine oocytes without BCB staining (controls) and BCB+ oocytes compared to BCB- oocytes. This is similar to our results from the second part of the study. However, our findings from the first part of the study, showed no significant difference between the maturation rate of high quality COCs stained BCB+ and BCB- (66% vs. 53%). We assume that the lower number of oocytes involved in the COC+/BCB- group in the first experiment might be the reason for this discrepancy.
A significantly lower maturation rate in the BCB- oocytes (28%) compared to the BCB+ and the control group (59% and 63%, respectively) registered in our study is in accordance with reports from Liu et al. (
15). Those authors observed the cumulus expansion in BCB- group compared to the control and BCB+ group and reported a significantly lower maturation rate in the BCB- group vs. the control and BCB+ group. Similarly, Lee et al. (
2) noticed higher complete cumulus expansion (p<0.05) of BCB+ oocytes, opposite to the cumulus expansion in BCB- oocytes. The matured BCB- oocytes in the current study (28%) are similar to data reported in the study of Egerszegi et al. (
16), who found that only 28.3% of the BCB- oocytes reached the metaphase-II maturation stage.
The research of Alvarez et al. (
17) showed that cumulus cells have an important role in oocyte maturation to metaphase-II stage, therefore, oocytes with compact cumulus mass are used for
in vitro maturation protocols. For this reason, oocytes with incomplete cumulus mass or <3 layers of cumulus cells, are usually discarded from further evaluation. We aimed to elucidate whether the BCB staining method of COCs with low morphological quality could aid in assessing their developmental potential. The results from the first part of our study showed that 56% have been stained BCB+, and 38% of them have matured, which leads to an assumption that the BCB staining method might be useful for selecting developmentally competent oocytes that have been morphologically classified as low quality. Although this rate (38%) registered in COC-BCB+ oocytes is lower compared to this maturation rate in high quality oocytes, it is statistically (p<0.01) higher than the lowest maturation rate (29%) found in the group with low quality colorless oocytes COC-BCB-.
To further evaluate the developmental potential of high quality and low quality oocytes stained with BCB, the next step will be to compare the blastocyst rate, in order to elucidate whether there will be significant difference between embryos obtained from oocytes with different quality and BCB staining.
CONCLUSIONOur findings indicated that COCs with high morphological characteristics have statistically higher developmental ability evidenced by higher maturation rate compared to the COCs with low morphological characteristics. Also, the BCB staining method might be useful for selecting developmentally competent oocytes that have been morphologically classified as low quality oocytes.
However, despite that the brilliant cresyl blue is a good indicator for COCs quality, the lack of a significant difference with the control group, leads to an assumption that it should not be used as an independent method for porcine oocyte selection, but as an additional method to the morphological classification. Combining those two methods can enhance the morphological quality evaluation of the porcine oocytes.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThis research was part of a project “Molecular study of the oocytes with different developmental competence” (FVMS-IPR-02), funded by the Faculty of Veterinary Medicine-Skopje.
AUTHORS’ CONTRIBUTIONSMD conceived the study, conducted the experiments and wrote the manuscript. TD, BA and NA collected the samples. MN was involved in statistical analysis of data. FPP, TD and MN contributed to data interpretation. TD, FPP, MB, LP and JL supervised the study, gave critical revision and contributed to the final version of the manuscript. All authors approved the final version of the study.

 10.2478/macvetrev-2023-0019
10.2478/macvetrev-2023-0019





