This descriptive study aims to examine the behavior of dogs diagnosed with hypothyroidism and the potential effect of hormonal treatment. Eight client-owned dogs with clinical hypothyroidism were evaluated using an adapted C-BARQ questionnaire, clinical description, and hematological analysis. Six of the dogs’ behavior was monitored for four months after the treatment initiation. The study found that excitation, whining, and urinating when left alone were frequently observed. Attention-seeking was also a common behavior reported by the owners. The biochemical analysis before the treatment had revealed elevated cholesterol, triglycerides, and alkaline phosphatase levels in the majority of dogs. The study emphasizes the need for prospective studies using a larger sample size to gain further insight into the relationship between hypothyroidism and behavior in dogs. Monitoring changes in behavior over time can provide insight into how thyroid dysregulation may contribute to the onset of certain behavioral patterns. Functional brain imaging and pathohistological brain analysis in dogs with long-term hypothyroidism are also required to confirm the effects of hypothyroidism on canine brain function.
Hypothyroidism is an endocrine disease with a prevalence of 0.2% to 0.8% that primarily affects middle-aged dogs (
1). The incidence of the disease appears to be similar between males and females, regardless of whether they are neutered or intact (
2). Thyroid hormones influence nearly every organ system in the body, and deficits can cause a wide range of clinical symptoms (
2, 3). The most common manifestations include lethargy, weight gain, hair loss, skin changes, and neuropsychiatric abnormalities (
4, 5, 6). Hematological and biochemistry findings may consist of normocytic, normochromic anemia, and elevations in cholesterol, and creatinine kinase levels (
5, 7). Thyroid hormones affect a wide range of metabolic activities at the cellular level, including lipolysis and mitochondrial oxygen demand (
8). Their primary mode of action on gene expression is through transcriptional regulation (
5). Diagnosis of hypothyroidism can be challenging due to the wide range of clinical signs, requiring a thorough evaluation of clinical signs, laboratory findings, and response to treatment. In addition to the diagnostic challenges, dogs diagnosed with hypothyroidism have been observed to experience changes in their behavior. While some authors have asserted that dogs with hypothyroidism may exhibit signs of aggression (
9, 10, 11), others have found no connections between this behavior and hypothyroidism (
12, 13). The thyroid hormone is known to encourage neuroprotection and control neurogenesis (
14). On brain imaging techniques in humans (
15, 16) and rats (
17), thyroid hormones alter neuronal differentiation, migration, and myelination. Based on the claims of previous findings, the current study hypothesized that hypothyroidism may contribute to behavioral changes in dogs. The aim was to examine the hematological, clinical, and behavioral changes before and during treatment in dogs diagnosed with hypothyroidism and to evaluate the potential effect of hormonal treatment on these parameters.
MATERIAL AND METHODSThe study included eight dogs of various breeds, consisting of four males and four females, aged between 4 and 15 years. The clinical examination, diagnosis, and treatment of dogs with hypothyroidism were conducted at the University Veterinary Hospital in Skopje. All examinations were performed after obtaining consent from the patient’s owner. Inclusion criteria required the presence of clinical hypothyroidism symptoms and low free thyroxine levels (fT4<0.8 ng/dl), as well as the absence of any other non-thyroidal disease that can affect the fT4 levels. None of the patients received any treatment that could potentially impact fT4 levels, such as trimethoprim/sulphonamide, glucocorticoids, phenobarbital, carprofen, ketoprofen, aspirin, and clomipramine, within the six weeks preceding the diagnosis (
18). The clinical examination encompassed various assessments including measuring the heart rate, conducting respiratory auscultation, determining the respiratory rate, evaluating the temperature, palpating lymph nodes, performing abdominal palpation, and assessing body score condition. Furthermore, the doctors evaluated each patient for neurological abnormalities. This involved conducting a detailed anamnesis, assessing posture, coordination, gait, postural reactions, and identifying any abnormal movements. During the clinical examination, the owners were asked specific questions to gather additional information. These questions focused on changes in behavior such as alterations in sleeping, excitation, appetite patterns, the frequency and onset of different vocalization patterns, as well as abnormal movements observed in the dog’s environment. All patients included in this study were without any serious health problems at the time of examinations. In addition, there was no evidence about other severe none-thyroidal diseases that could affect the fT4 levels (gastrointestinal, neurological, endocrine, neoplastic, respiratory, urological, hepatic, infectious, etc.) (
19).
The selected patients received Levothyroxine therapy (
per os) at a dosage of 0.02 mg/kg, twice a day. Hematological, biochemical, and fT4 follow-up assessments were conducted one month after the treatment in all the patients included. The questionnaire follow-up assessments were carried out four months after the initial treatment, involving a total of six patients, comprising four males and two females. Two owners were excluded from the follow-up questionary analysis as the fourmonth time frame had not elapsed since the initial diagnosis. Free thyroxine levels were measured to diagnose hypothyroidism and blood samples were collected and analyzed immediately for both fT4 levels, complete blood count, and biochemistry analysis. The method used for measuring fT4 levels was chemiluminescent fT4a assay (Immulite 2000 Veterinary Free T4, Siemens Healthcare Diagnostics). Exigo H400 (Boule Diagnostics AB, Sweden) was used for testing whole blood, while ChemWell®-T (Awareness Technology, INC, USA) was used for biochemical analyses. The hematology, biochemistry, and fT4 values at the beginning (T0) and one month after treatment (T1) were presented as mean and standard deviation. Comparisons were made by using Wilcoxon Matched Pairs Test.
The owners were requested to complete a modified version of the Canine Behavior Assessment and Research Questionnaire (C-BARQ) (
20) to assess their pet’s behavior both prior to (T0) and four months into the treatment (T1). This study employed a questionnaire specifically tailored for owners, concentrating on alterations in their pet’s behaviors. The survey was conducted via telephone, and the adapted questionnaire encompassed questions from the original version organized into four distinct domains: aggression, fear, anxiety, attention, and excitability. Within each category, there were descriptive questions that were verbally clarified to the owners, and intended to define specific behaviors. To assess aggression, the following questions from the C-BARQ were utilized: stranger-directed aggression, owner-directed aggression, as well as familiar and unfamiliar dog-directed aggression. In terms of fear and anxiety, the questionnaire included questions regarding non-social fear, strangerdirected fear, and dog-directed fear (familiar and unfamiliar). For evaluating excitability, the questions focused on the dog’s response to everyday encounters, such as going for a walk, greeting owners after their absence, interacting with guests in their environment, and reacting to other dogs both inside and outside their environment. Attention-seeking behavior was assessed through questions concerning the tendency to follow owners in their environment, the frequency of staying in close contact, the reaction to showing affection to other dogs and people, and the urge and frequency of seeking affection from the owner in a calm environment among others. The intensity of these behaviors was indicated on a scale ranging from 1 (low intensity) to 4 (high intensity). To determine the frequency of each descriptor, the median was calculated from the sum of each category. This approach was deemed more appropriate given the limited number of patients included in the study. In addition to the survey, there were 19 questions regarding specific clinical signs extracted from the questionnaire. The prevalence of specific clinical signs was determined by calculating the percentages based on the number of owners who reported observing them. The specific questions regarding clinical signs pertained to appetite changes, abnormal movements, frequency and occurrence of micturition in their environment, abnormal behaviors, vocalizations, etc. Only the most frequently reported clinical signs among the patients are presented in the results in
Figure 1.
RESULTS
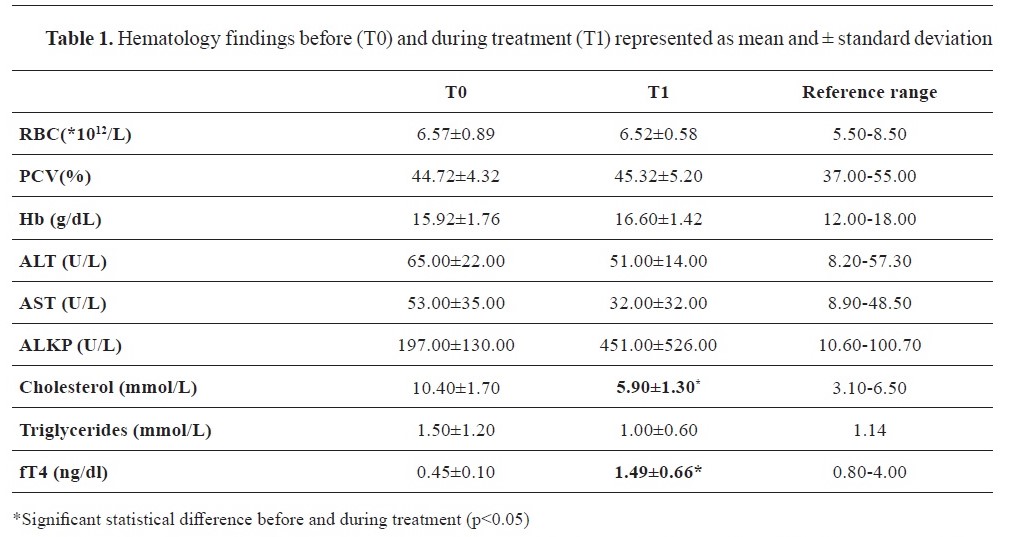
Before therapy (T0), the serum fT4 levels were 0.45±0.10 ng/dL, while after 1 month of therapy (T1), the fT4 levels significantly increased to 1.49±0.66 ng/dL (p<0.05) (
Table 1). In the hematological findings, two patients exhibited normochromic normocytic anemia that remained unresolved. Biochemistry results presented in
Table 1 revealed elevated alkaline phosphatase in 7 patients, while ALT was above the normal reference range in 3 patients. A decrease in AST and ALT parameters was presented whereas ALKP showed an increasing tendency (T1,
Table 1). Treatment with Levothyroxine also led to a clear reduction in cholesterol levels, while triglyceride levels dropped within a normal reference range in 6 out of 7 patients (T1,
Table 1).
Before the diagnosis of hypothyroidism, the medical records showed that 5 out of 8 patients were obese, and 2 patients had alopecia with no other visible skin abnormalities. One patient had ophthalmic changes, and another had a history of high neonatal mortality, which later resulted in sterility. During the follow-up, the dermatological clinical presentation improved, but the sterility, obesity, and ophthalmic changes remained unresolved.
Figure 1 presents specific clinical signs before (T0) and 4 months after therapy (T1). The patients exhibited a decrease in profuse salivation, whining, urinating when left alone, as well as behaviors such as following shadows, bright spots (indicating abnormal behavior), and other external stimuli. Restlessness, excitement, and high energy levels were the most common symptoms among the patients after 4 months of therapy in this case series. During the examination, the majority of the owners reported to the doctors that the only discernible difference in their pets’ behavior before treatment was decreased activity and an increased frequency of sleeping.

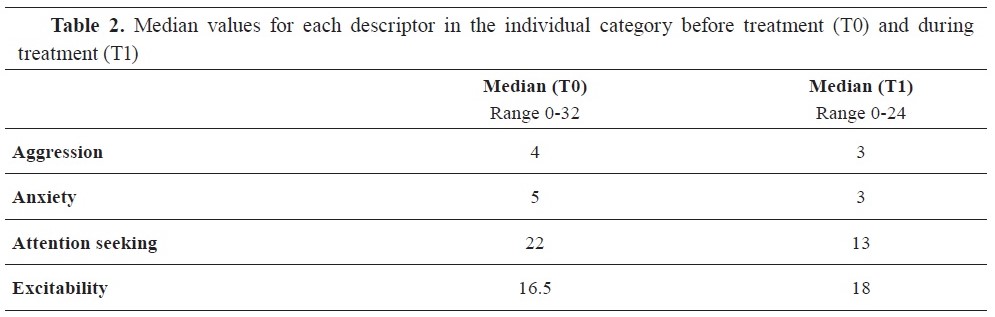
Results of the questionnaire before therapy showed that aggression and anxiety were reported less frequently than other behavioral categories (
Table 2). Attention-seeking behavior had the highest frequency and was noted as the most intense behavior, while excitability was recorded at half its maximum range (
Table 2). After four months of therapy, results revealed a change in the ranking of attention-seeking and excitability behaviors, with excitability being reported as the most frequent while attention-seeking was less prominent. Aggressive behavior and anxiety were reported even less frequently than before (
Table 2).
 DISCUSSION
DISCUSSIONThe pathophysiology of hypothyroidism in dogs is complex, involving interactions between thyroid hormones and various physiological and behavioral processes in the organism (
5, 6, 13). Due to the unavailability of diagnostic tools in everyday clinical practice and expensive laboratory methods specifically designed to accurately measure the serum concentration of thyroid hormones, routine clinical work often lacks the means for definitive confirmation of hypothyroid disease. Consequently, varieties of diagnostic approaches were employed to assess the presence of hypothyroidism in the examined patients.
During the clinical examination, all dogs presented a normal habitus. However, further observations revealed several noteworthy findings that served as indicators of diverse subclinical and clinical symptoms in hypothyroid dogs. These included obesity in five patients, symmetric noninflammatory and non-pruritic hyperpigmented alopecia in two dogs, as well as unspecific ocular disease, and sterility in one dog. Routine hematology testing and biochemistry profiling can be helpful in evaluating individual cases with suspected hypothyroidism, though with small diagnostic relevance (
21). In this study, normocytic normochromic anemia was identified in two patients, while a consistent finding was the significant elevation of serum triglycerides and cholesterol levels. Additionally, mild increases in alanine aminotransferase (ALT) were observed in dogs with hepatic impairment caused by the low metabolic rate in hepatocytes (
5). However, we also observed an increase in alkaline phosphatase, which is not consistent with previous studies and may be attributed to non-thyroid-related processes (
5). These findings highlighted the importance of recognizing and assessing these symptoms in diagnosing hypothyroidism in dogs.
To further assess the thyroid function, the bioactive free fraction of thyroxine hormone (fT4) was analyzed using a highly sensitive chemiluminescent immunoassay method. Of all the thyroid hormones, measurement of free T4 by equilibrium dialysis (fT4d) is the single best diagnostic test for canine hypothyroidism, but the analyses are technically demanding (involve the use of radioisotopes) and are expensive to perform, with limited availability (
22). Chemiluminescent analyses of the fT4 can be affected by non-thyroidal diseases and several therapeutic drugs (
18, 19). In order to avoid this inconvenience, only patients without moderate to severe non-thyroidal disease or no previous treatment with thyroid-effective drugs were included. In a recent study, a good correlation between fT4 by immunoassay and fT4 by equilibrium dialysis method was found (
23). With this approach, the possibilities of false low levels of fT4 are minimal.
The results revealed a wide variation in the serum concentration of fT4 among all dogs, but consistently, all values were below the established reference minimum. The treatment protocol involving thyroid hormone supplementation yielded the expected results, leading to improvements in the clinical condition of the patients, correction of lipid levels, and positive changes in estimated behavioral parameters.
Recent research has dispelled the belief that hypothyroidism is associated with aggressive behavior in dogs and instead suggested an increase in activity as the most characteristic symptom (
12, 13). This study also signified that hypothyroidism in dogs is not associated with aggressive behavior but is associated with frequent attention-seeking and excitation. The results have shown that hormonal therapy can reduce attention-seeking behavior, together with the improvement of clinical examination findings. It is also important to consider small signs such as odd behaviors, whining, tremors, and urinating when left alone when diagnosing and treating hypothyroidism in dogs. Our study revealed that owners reported a greater degree of change when responding to the questionnaire compared to the information gathered through the doctor’s anamnesis. In contrast to human medicine, diagnosing and determining the prevalence of hypothyroidism in dogs remains challenging. To advance our understanding of disease-related behavior changes in dogs with hypothyroidism, it is imperative that we adopt a more comprehensive research approach, which would offer greater confidence in our findings. For instance, we could perform pathohistological studies to explore structural changes in the brains of dogs with clinical hypothyroidism or utilize functional magnetic resonance imaging (fMRI) (
15, 16, 17). These studies could yield more definitive evidence about the neurological effects of the disease, and prove beneficial for both clinical practice and academic research.
One limitation of our study was the small sample size, which may limit the generalizability of our results. Additionally, relying on owner-reported data through the questionnaire may introduce response bias. Further studies with larger sample sizes and more objective measures of behavior are needed to confirm our findings.
CONCLUSIONDespite the study’s limitations, the findings indicate that monitoring hormonal concentration and clinical symptoms alone may not be sufficient for managing hypothyroidism in dogs. It is also crucial to pay close attention to any behavioral changes and obtain a detailed anamnesis. This study underscores the significance of vigilant observation of both clinical and behavioral indicators for the successful management of this disease.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSWe thank the doctors from the University Veterinary Hospital in Skopje for their collaboration and providing additional data.
AUTHORS’ CONTRIBUTIONSEM conceived and designed the study, performed the clinical examinations, and collected the data. EAP contributed to the interpretation of the results and provided guidance throughout the study. MKj conducted the statistical analysis and contributed to the data interpretation. IC drafted the manuscript and revised it critically for important intellectual content. All authors reviewed and approved the final version of the manuscript.

 10.2478/macvetrev-2023-0021
10.2478/macvetrev-2023-0021


