Myxomatous mitral valve disease (MMVD) is one of the most common heart diseases in Cavalier King Charles spaniel (CKCS) dogs. The American College of Veterinary Internal Medicine (ACVIM) uses clinical, echocardiographic, and radiographic criteria to diagnose the disease, but measurement of vertebral left atrial size (VLAS) provides a simpler assessment. The aim of this study was to determine VLAS values in MMVD cases of CKCS and to investigate their clinical significance at different MMVD stages. Eighteen CKCS dogs of both sexes, different ages and weights, and different MMVD stages (6 at B1 stage, 6 at B2 stage, and 6 at C stage) were included in the study, as well as 6 healthy CKCS as control group A. We performed clinical, radiological, and echocardiographic examinations. VHS and VLAS values were significantly higher in the MMVD group than in the control group (p<0.001). VLAS showed high diagnostic accuracy in the detection of LA enlargement (area under the curve [AUC]: 0.98, cutoff ≥ 2.25, sensitivity: 88%, specificity: 100%, p<0.001). We also found high positive correlations between the VLAS and other values (LA /Ao, LVIDDn, and VHS) (r=0.88, r=0.88, and r=0.86, respectively) (p<0.001). It has been concluded that a VLAS value ≥2.25 can provide a meaningful diagnosis of left atrial enlargement in dogs with MMVD CKCS.
Approximately 10% of patients presenting to the veterinary clinics have heart disease, and 75% of these have myxomatous mitral valve disease (MMVD) (
1, 2, 3, 4). Dogs of the CKCS breed have a strong tendency to develop this disease (
1, 5, 6, 7, 8). Hemodynamic changes due to mitral regurgitation may develop after a long preclinical period and lead to congestive heart failure and fatal pulmonary edema, especially when associated with enlargement of the left atrium (
1, 2, 9, 10, 11, 12). Various echocardiographic and radiographic criteria based on the American College of Veterinary Internal Medicine (ACVIM) criteria are used to diagnose and grade the disease (
3, 9). Echocardiography is the gold standard noninvasive method for assessing left atrial size (LA) in dogs with MMVD, but it is not always practical because of limited accessibility, examination costs, and expert examination (
9, 10, 12). Therefore, researchers have reported that there is a need for accurate radiographic criteria that can detect the disease more easily (
9, 10, 11, 12). These criteria are more readily available than echocardiography, less costly, and may serve as gold-standard procedures for the diagnosis of left heart failure (
9, 10, 11, 12).
VLAS has become a valuable tool as a radiological index to detect enlargement of the left atrium (LAE) in dogs with MMVD (
9, 10, 11, 12). Different studies have proposed different VLAS cut-off values for LAE for dogs with MMVD (
9, 10, 11, 12). Our aim was to determine the diagnostic and the optimal cutoff of VLAS values and evaluate their clinical significance in different stages of MMVD in CKCS dogs.
MATERIAL AND METHODSEighteen CKCS dogs with MMVD participated in the study. They were divided into three subgroups (B1, B2, and C) of six dogs each, according to ACVIM guidelines (
3). In addition, six healthy CKCS dogs were included in a control group (A). The ACVIM diagnostic criteria of a murmur density ≥3/6, a left atrial aortic ratio (LA/Ao) ≥1.6, a left ventricular diastolic internal diameter (LVIDDn) ≥1.7, and a vertebral heart score (VHS) > of 10.5 were used to classify dogs in stage B2. Dogs with congestive heart failure were classified as stage C. We recorded the body weight, age, and sex of all dogs in both groups.
Having obtained informed consent from the patient’s owner, we performed all the examinations. The study was approved by the Veterinary Faculty, Istanbul University-Cerrahpasa on March 16, 2021 (No. 2021/15).
Echocardiographic and Doppler examinations were performed with the SIUI Apogee 3500 V Doppler ultrasound machine and the multifrequency P3F14C Cardiac Phased Array Probe (Shantou Institute of Ultrasonic Instruments, China). The values of LA/Ao (left atrial to aortic root ratio) were obtained from B-mode and left ventricular internal diameter in diastole (LVIDD) was measured in M-mode at the right parasternal window short axis view according to previously described echocardiographic measurement techniques (
13, 14). Normalization of left ventricular internal diameter (LVIDDn) was performed according to the formula given by the investigators (LVIDDn=LVIDd (cm)/body weight (kg)0.294) (
15).
Right-side lateral chest radiographs were obtained from each patient using a digital radiographic unit SMS-CM -N (EcoRay, Korea). The vertebral heart score (VHS) of each patient was calculated as the sum of the long and shortaxis distances drawn from the cranial border of the fourth thoracic vertebra according to the technique developed by Buchanan and Bucheler (
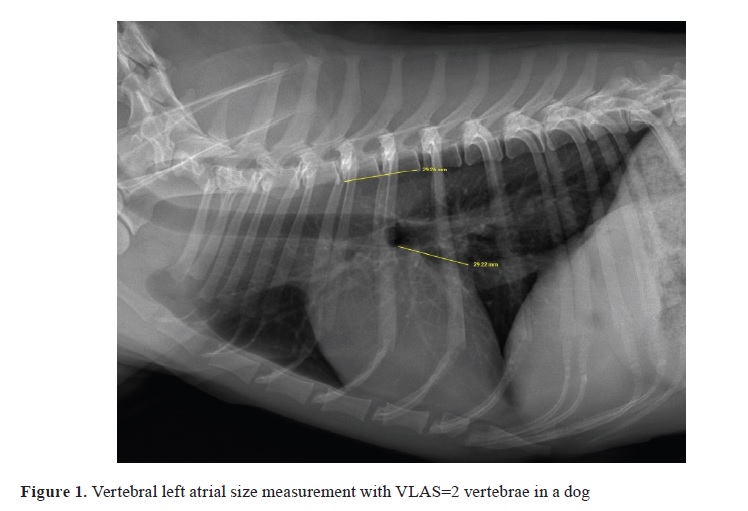
16). The long axis was measured from the ventral border of the carina to the apex of the heart, and the short axis, perpendicular to the long axis, was defined as the line with the maximum dimension of the heart in the central third region including the right atrium and left heart chambers. The vertebral left atrial size (VLAS) of each patient was measured on a lateral radiograph by drawing a line from the center of the most ventral aspect of the carina to the most caudal aspect of the LA where it intersects with the dorsal border of the caudal vena cava. The same line length was drawn beginning at the cranial edge of the fourth thoracic vertebra and expressed in vertebral body units to the nearest 0.1 vertebra as VLAS (
9, 10, 11). To assess the measurement variability of radiographs, they were evaluated by intraobserver (G, R.) and interobserver (S, H., M, S.). The extent of pulmonary edema was determined by the extent and density of alveolar opacities on the chest radiograph and graded as 0, +1, +2, and +3 according to severity.
The SPSS statistical software package (version 28 Windows, IBM Corporation, NY) was used for statistical analyses. The Shapiro-Wilk test was applied to determine whether the data sets were normally distributed, and the normally distributed variables were expressed as mean and standard deviation. When a present normal distribution was determined, one-way ANOVA and Duncan multiple comparison tests were performed to compare the healthy, B1, B2, and C groups. The homogeneity of variance was checked with Levene’s test. The area under the receiver characteristic curve (AUC) was used to evaluate diagnostic accuracy. The optimal clinical cutoff value for the radiological scores was determined to be the value with the highest Youden index. The relationships between VLAS, LA/Ao, LVIDDn, and VHS were evaluated using Pearson’s correlation coefficient. Intraobserver and interobserver agreement were assessed by an intraclass correlation coefficient (ICC) for absolute agreement. For all analyses performed, a p<0.05 was considered statistically significant.
RESULTSGroup A consisted of three female and three male dogs. Group MMVD included seven female and eleven male dogs. The average age of groups A, B1, B2, and C were 4.92±2.9, 6.83±1.94, 8.15±2.82, and 9.5±2.42 years (mean±SD), respectively. Body weights were 8.13±2.01, 9.16±2.69, 11.08±1.62, and 10.33±2.6 kg in groups A, B1, B2, and C, respectively.
The study showed an increase in pulmonary edema (grade +1, +2, and +3 in groups B1, B2, and C, respectively) and heart murmurs (>3/6 in groups B2 and C) depending on the disease stage.
Patients with stage C heart disease had significantly higher echocardiographic values (LA/Ao and LVIDDn) than the other groups (p<0.001) (
Table 1).
Group C showed significantly higher VHS and VLAS (p<0.001) than the other disease stages (
Table 1). The VLAS was 2 vertebrae in group A, 2.4 vertebrae in group B1, 2.68 vertebrae in group B2, and 3.43 vertebrae in group C (
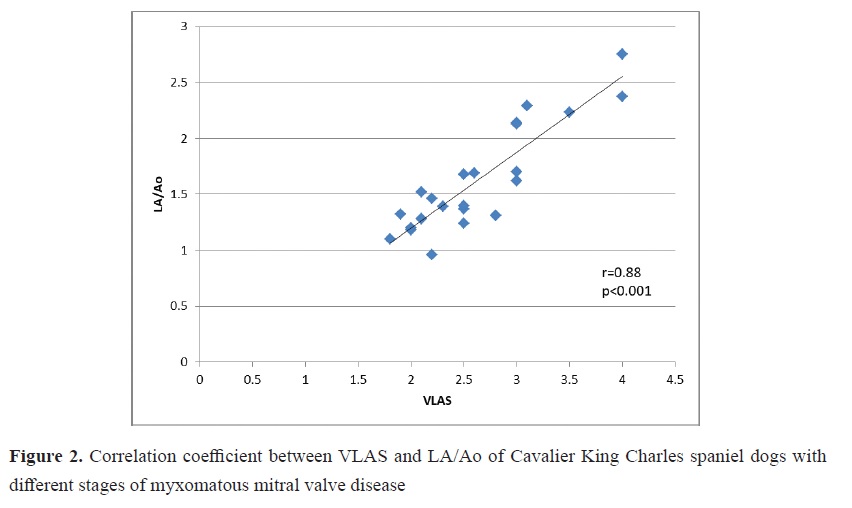
Fig. 1). In addition, there were high positive correlations between the VLAS and LA /Ao (r=0.88), VLAS and LVIDDn (r=0.88), and VLAS and VHS (r=0.86) (
Fig. 2).

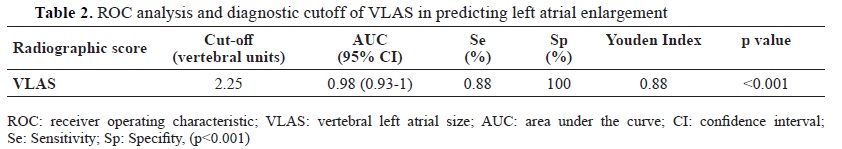
The optimal cut-off for VLAS to detect LA enlargement was ≥2.25 (p<0.001) (
Table 2). There was high intraobserver and interobserver measurement agreement with intraclass correlation coefficient (ICC) of 0.98 and 0.92, respectively.
 DISCUSSION
DISCUSSIONIn dogs of the CKCS breed, MMVD is the most common cardiovascular disease, with a prevalence of 50% in dogs older than 4-6 years (
6, 8, 17, 18). We also found similar results, namely that the incidence and severity of the disease in animals increase from 5 years of age.
Because patients may be asymptomatic or preclinical for many years during the progression of MMVD, rapid progression may be seen in other dogs (
2, 3, 5, 9). When MMVD valve disease progresses, congestive heart failure develops, leading to dyspnea, syncope, cyanosis, and even death (
19). Among other changes, hypertension, and cardiac arrhythmias may also be observed (
19). In our studied population, we observed that the severity of dyspnea, cyanosis, and heart murmurs increased with the severity of the disease.
Weaker LA function may be observed in dogs with MMVD with increased LA volume (
2). The distance between the vena cava and the carina increases when we observed the enlargement of the heart. Elevation of the trachea also increases this distance. Cardiac enlargement is one of the differentials of tracheal elevation, alongside heart base tumors, tracheal lymphadenopathy, and esophageal diseases. VLAS reflects enlargement of both the left atrium and the left ventricle (
11). Similarly, VLAS is thought to provide valuable diagnostic information in dogs with heart murmurs (
10).
This information helps distinguish between cough and dyspnea, a conundrum for veterinarians, to determine if they are due to respiratory disease or cardiogenic pulmonary edema (
10). In addition, this can be used to monitor disease progression or treatment success (
9).
Although there are clinical, echocardiographic, and radiological ACVIM criteria used to diagnose the disease, VLAS measurement, which provides a simpler assessment of the disease compared with analyses that require expertise, such as echocardiography, and has recently gained prominence, has provided a perspective that may allow clinicians to more readily suspect the disease and use other diagnostic tools, such as echocardiography, to confirm the diagnose (
9, 10, 12).
Kim et al. (
9) reported that regular monitoring of VLAS as an additional follow-up index for MMVD would help detect changes in left atrial size. Mikawa et al. (
11) also found that VLAS increased significantly with disease severity. Malcolm et al. (
10) found that VLAS ≥2.3 vertebrae is a reliable indicator for identifying left atrial enlargement. They also observed a positive correlation between VLAS and LA:Ao short and long-axis ratios (
10). Vezzosi et al. (
12) also reported that the best cutoff value for detecting LA enlargement was 2.2, and a VLAS value of 2.4 was the best predictor of moderate to severe LA enlargement. In their study, Mikawa et al. (
11) discovered a positive correlation (r=0.68) between VLAS and LA/Ao. They found that the optimal VLAS value for ACVIM grade B dogs with LA/Ao ≥1.6 on the short axis was 2.5, with a sensitivity of 62%. VLAS had high accuracy in detecting enlargement of the left heart and in discriminating stage B1 - B2 MMVDs (
10, 11, 12). Similarly, they discovered that VLAS also correlated with LVIDDn, which echocardiographically indicates enlargement of the left ventricle (
9, 12). Mikawa et al. (
11) reported that comprehensive cardiac monitoring is required when the VLAS is ≥2.5, and when it is ≥3.1, it indicates the development of stage B2 MMVD, even if echocardiography was not performed.
Cavalier King Charles spaniel dogs have been used in a limited number of studies and it has been noted that new studies on breed-specific values should be conducted by researchers because changes may occur in any patient group (
9, 10, 20). In our studied population, we found that VLAS increased proportionally to disease severity and were significantly higher in the MMVD group than in the control group (p<0.001). VLAS showed high diagnostic accuracy in detecting LA enlargement (area under the curve [AUC]: 0.98, cutoff ≥ 2.25, sensitivity: 88%, specificity: 100%, p<0.001). We also found high positive correlations between the VLAS and LA /Ao (r=0.88), LVIDDn (r=0.88), and VHS (r=0.86) (p<0.001). This is consistent with reports from researchers who have found a high positive correlation between VLAS scores and LA /Ao, LVIDDn, and VHS (
9).
CONCLUSIONAs a result of this study in Cavalier King Charles spaniel dogs, a cut-off VLAS value ≥2.25 in dogs with MMVD was found to be a cost-effective, easy-to-use, and reliable method to detect left atrial enlargement and monitor disease. However, the atrial enlargement does not always point in the same direction because of the overall structure of the heart, pulmonary edema, effusion, mass formation, and so on. Considering the limitations of radiographic diagnosis in the presence of additional diseases and the possible inclusion of breed-specific differences, new studies on this topic are needed.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThe authors are grateful to Prof.Dr. Bulent Ekiz from the Department of Animal Breeding and Husbandry for his help with the statistical analysis. This study was supported by the Scientific Research Found of the Istanbul University-Cerrahpaşa with project number: TSA-2021-35735.
AUTHORS’ CONTRIBUTIONSRG designed, analyzed the data and wrote the study. RG, HS, and SM collected the patients, made radiography and echocardiography.

 10.2478/macvetrev-2023-0023
10.2478/macvetrev-2023-0023



