Canine parvovirus (CPV) and canine distemper virus (CDV) are the most widespread viral pathogens in young dogs worldwide, causing significant morbidity and death (
1). Canine distemper disease caused by CDV belongs to the genus of Morbillivirus in the family Paramyxoviridae and affects systemic, respiratory, cutaneous, bone, and neurological systems in a broad diversity of mammalian hosts (
2). On the other hand, CPV belongs to the genus Parvovirus of the Parvoviridae family (
3). CPV infects rapidly dividing cells, including the bone marrow, lymphoid tissue, and gastrointestinal tract, and causes common clinical signs including lethargy, vomiting, fever, and bloody diarrhea within 3–7 days after infection (
4). Myocarditis development, which is among the critical and lethal findings in juvenile dogs, has been reported in both viral diseases, especially CPV (
5).
The most important factor affecting the disease mortality rate (parvoviral enteritis and distemper) is the immune status of puppies. Vaccination is widely recognized as the primary and most effective approach to safeguard dogs against viral diseases (
6). In fact, vaccination represents the sole means of prevention (
6), and young animals acquire protection against the disease through vaccination and the presence of maternal antibodies (
7). It has been reported that dogs become immune to the disease when antibody titers are 1:80 and above following vaccination (
8). Nevertheless, several factors can influence the efficacy of vaccination, such as variations in vaccine protocols, challenges in differentiating between maternal antibodies and vaccine-induced antibodies, and potential immune system activation due to environmental exposure (
9).
Vitamin D is a classically well-known regulator for skeletal function; however, it has a role beyond that. Notably, vitamin D has an important effect on the immune system through the modulation of innate and adaptive immunity and the regulation of inflammation (
10). The immunomodulatory effects of vitamin D are attributed to the increased expression of vitamin D receptors in various immune cells, particularly those that express 1α-hydroxylase, an essential protein involved in the synthesis of the active form of vitamin D (
11, 12). These effects include the modulation of T-cell response, shifting from a Th1 to a Th2-mediated cell response (
13), as well as the regulation of Treg cell differentiation (
14). In the aforementioned studies, it was observed that vitamin D administration, together with vaccines, increased antibody response against different agents in human studies (
15, 16), likely because vitamin D important role in the regulation of immune response is related with vaccination too.
Similar to vitamin D, the probiotic bacteria have an effect on the immune system (
17, 18). They have a direct effect in reducing the access of pathogenic bacteria-related structures to the intestinal lymphoid tissue by regulating the harmful bacteria population in the intestinal microbiota. Moreover, they provide indirect effects through short-chain fatty acids, a microbial product formed in the intestines (
19). Animal models provide evidence indicating that the resident gut microbiota plays a role in shaping antiviral defenses and influencing the outcome of viral infections.
The studies involving germ-free mice have shown increased susceptibility to various infections, including influenza, highlighting the modulatory effect of gut microbiota (
20). Furthermore, probiotics exert regulatory effects on the immune system, allowing for the simultaneous coordination of the immune response following vaccination (
21). It has been reported that
Bifidobacterium species predominantly have positive effects on the immune response after vaccination, especially in infants. Thus, increased intestinal colonization of
Bifidobacterium spp. may encourage greater efficacy of vaccines by improving immunologic memory in infants (
22) and by increasing various vaccine-specific IgG and IgA in saliva with supplementation of
Bifidobacterium animalis subsp
. lactis and Lactobacillus paracasei subsp
. paracasei in influenza vaccination (
23).
There is currently a lack of research examining the effects of probiotics or vitamin D supplements on the immune system of vaccinated puppies. Therefore, the objective of this study was to investigate the impact of orally administered vitamin D and a combination of
Bifidobacterium animalis subsp.
lactis and vitamin D on the immune response against CPV and CDV following vaccination.
MATERIAL AND METHODSDogs and groups compositionA total of 21 crossbred dogs between 55–65 days old of both sexes were used for vaccination protocol at Faculty clinics. All animals were enrolled on a voluntary basis after obtaining the consent and information form from the animal owners, and this study was approved by the local ethical committee of the Aydın Adnan Menderes University, number 645831101/2019/095.
Following routine clinical examinations, antiparasitic treatment was first performed with an antiparasitic agent (Caniverm, Biovet, Turkey). After the antiparasitic treatment, the vaccination protocol was conducted according to the manufacturer’s instructions (Vanguard 5L4, Zoetis, Turkey). The vaccine contained frozen and dried attenuated strains of Canine Distemper virus (CDV), Canine Adenovirus Type 2 (CAV 2), Canine Parainfluenza virus, and Canine Parvovirus (CPV), as well as inactivated cultures of
L. canicola,
L. grippotyphosa,
L.
icterohaemorrhagiae, and
L.
pomona.
Within the scope of the research, three groups were formed. Vaccination was administered to all animals in each group according to the instructions provided by the manufacturer, with a 3-week interval between each vaccination. The animals in Group 1 underwent the vaccination protocol alone, while the animals in Group 2 received a solution containing both the vaccination and Vitamin D. In Group 3, the animals were administered a solution containing a combination of B.
animalis subsp.
lactis and Vitamin D
3 alongside the vaccination protocol. For the combination of B.
animalis subsp.
lactis and Vitamin D
3 in Group 3, Bi-bac D
3 drop (Consentis, Denmark) was used, while in Group 2, only Devit 3 drop (DEVA, Turkiye) containing only Vitamin D
3 was used. In Group 2, dogs received 6 drops of Devit 3 per day (1 drop contains 800 IU of Vitamin D
3), while in Group 3, animals were given 12 drops of Bi-bac D
3 (1 drop contains 400 IU of Vitamin D
3 and 1x10
5 CFU of
B. animalis subsp.
lactis) during the vaccination protocol.
Blood sample collection and processingBlood samples were collected from the cephalic vein on the day of vaccination to determine antibody titers against CPV and CDV. This process was repeated in three-week intervals, three times in total. The collected blood samples were centrifuged with an anticoagulant at a speed of 3,000 rpm for a minimum of 10 minutes to separate the plasma. The obtained plasma samples with antibody titers were then stored at a temperature of -20 °C until further analysis.
Antibody analysisThe antibody titer against CPV and CDV was measured by immunochromatographic assay. Separate test kits with the same procedure were used according to the manufacturer’s instructions (Bionote, Vcheck, Korea): 1) 5 μl of plasma were added into the separated assay diluent tubes of CPV and CDV using disposable capillary tube and were gently mixed, 2) 100 μl of the mixed sample were added to the sample wells of the test cartridges, and 3) the test was started on the device, and after 10 minutes, the results were read.
The results of the antibody titers were documented as numerical values rather than the categorization procedures determined by the manufacturer. All data were transformed to a base of Log
n to perform statistical analysis. Similarly, post-transformation cut-off values of antibodies were performed as 1:80, 4.27 for parvovirus and 1:32, 3.23 for distemper virus to evaluate immune status after vaccination in both diseases.
Statical analysisStatistical analyses were performed using the SPSS 22.0 program (IBM, America). To ensure normal data distribution, Log
n transformation was used. One-way variance analysis (ANOVA) was used to determine the differences between groups of transformed data, and a post-hoc Tukey test was performed to compare the means of the groups when significant effects were found. The logarithmic values obtained were tabulated with their geometric mean and 95% confidence interval limits. A value of p<0.05 was considered statistically significant.
RESULTSClinical findingsNo pathological clinical findings were noted during the vaccination period or in follow-up. However, there was a distinct difference in the habitus of the animals in Group 2, including increased appetite and brighter hair coats compared with the other groups. No post-vaccination anaphylaxis or vaccine site sarcoma was encountered in the animals.
Antibody titersThe distribution of antibody titers and antibody levels against CPV and CDV are shown in
Table 1 and
Fig. 1 and
2, respectively.
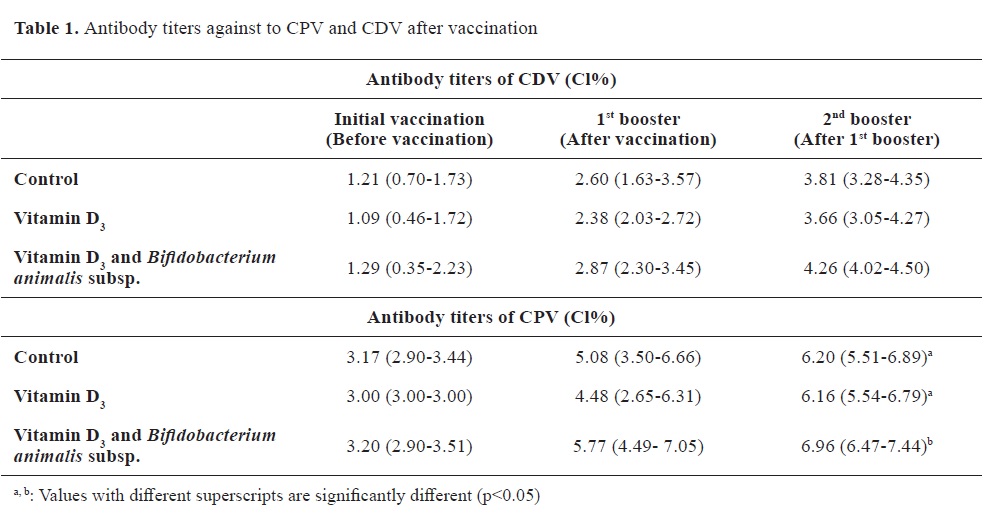
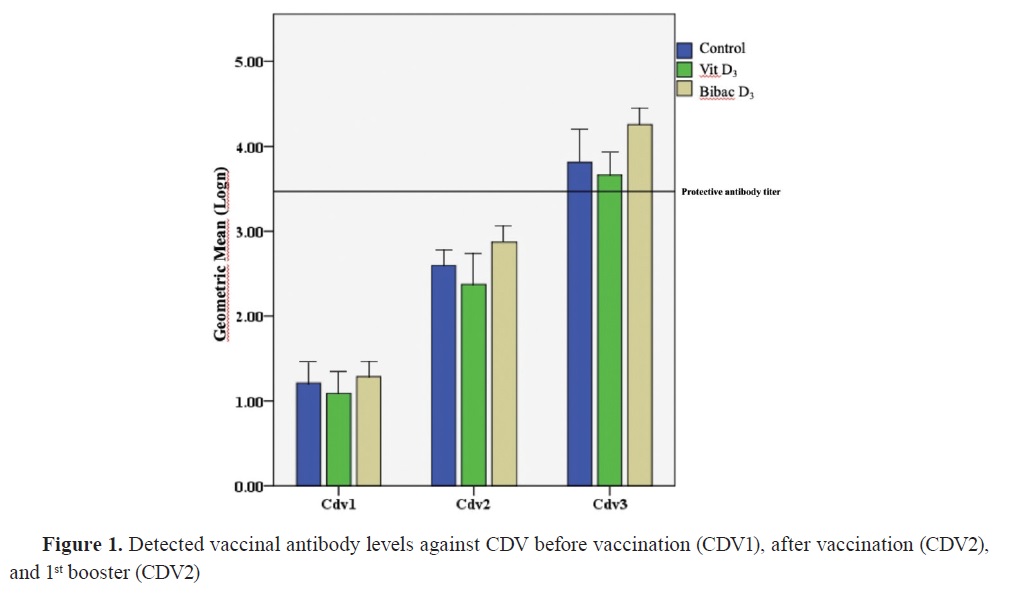

The antibody titers against CDV were nonsignificantly different at the initial vaccination time between the groups. This lack of significant difference was also observed between the first and second booster vaccination. Similarly, the antibody titers against CPV showed no significant differences at the initial vaccination time. Furthermore, there were no significant differences between the groups during the sample collection at the first booster vaccine appointment. However, the antibody response against CPV during the second booster sampling in Group 2 was significantly lower than the control, and vitamin D alone groups.
During the follow-up period for general vaccination against CDV, it was observed that the animals reached the cutoff value of antibodies specified by the manufacturer during the second booster vaccination (
Fig. 1). Regarding the antibody levels against CPV in the groups, it was determined that a satisfactory level of antibody titer was achieved with the first booster dose (
Fig. 2).
DISCUSSIONBesides its classical effects, the most important effects of vitamin D are known to be on the immune system (
10). Vitamin D receptors are found on many natural and acquired immune cells, including B lymphocytes, CD4 and CD8 T lymphocytes, dendritic cells, macrophages, and neutrophils (
10, 24). In particular, in vitro studies show that vitamin D suppresses TLR4 production and produces an anti-inflammatory activity by suppressing the production of proinflammatory cytokines from leukocytes (
11, 12, 25). At the cellular level, by modulating the differentiation in vitamin D, CD4+ levels support Th2 levels to improve humoral immunity (
13). Moreover, it helps to reduce T-cellbased autoimmunity by restricting Th1 and Th17 levels (
26). Thus, vitamin D is targeted by the immune system because it can both potentiate the innate immune response and restrict the adaptive system, so it can modulate vaccination response.
In the current study, there was no significant difference observed in the antibody levels against CDV in dogs that received vaccination along with vitamin D. When considering the follow-up vaccination protocol in the study, it was found that there were insignificant logarithmic increases in the duration of immunity and antibody levels in the subsequent weeks. In addition, not measuring vitamin D levels is considered to be one of the limiting factors of the study. As for antibody levels against CPV, it was determined that the dogs in the groups reached sufficient antibody titer at the time of the first booster. Notably, the antibody levels of the animals in the group administered with Bi-bac vitamin D
3 were significantly higher than the control and vitamin D alone groups at the time of the second booster dose. However, there was no significant difference between the vitamin D and the control group, indicating that vitamin D administration with vaccination is not sufficient alone for generating a significantly higher increase in antibody titers. This finding should be supported by further studies including larger populations.
Physiological and biochemical studies on
Bifidobacterium species based on different microbiological analyses have discovered that this bacterial family has a positive effect on the host immune system (
17, 18). It has been determined that the
Bifidobacterium family is responsible for the first colonization among commensal bacteria in the neonatal period for many species (
27). In this context, their generally recognized effect is the development of the immune system, the production of the mucus barrier, and the synthesis of glycans useful for the host in the intestinal lumen (
28). Another study related to influenza revealed that
Bifidobacterium species regulate humoral and cellular immunity through the Th1/Th2 balance (
29).
In recent times, significant advancements have been made in vaccine technology, along with a growing understanding of the role of microbiota in shaping the immune system. These changes in microbiota are recognized for their potential as endogenous adjuvants, further enhancing the immune response (
30). It is reported that the beneficial association of
Bifidobacterium genus in infants is due to the regulation of higher levels of immunoglobulin A secretion from plasma cells (
31) memory B-cells (
32), and enhancing IgA levels in salivary IgA (
33). Additionally, Huda et al. (
22) emphasized that the use of
Bifidobacterium species, together with vaccination against certain diseases, mediates significant increases in immunity as well as CD4 and CD8 T-cell proliferation. In our limited study, it was found that the antibody titers of the group administered
Bifidobacterium and vitamin D
3 (Bi-bac D
3) after the first booster period with multivalent vaccination were significantly higher than control and vitamin D alone groups. While this situation was parallel with other studies conducted in human medicine and laboratory animals, our study found that the respective relation was not similar for distemper disease antibodies. The magnitude of the immune response following multivalent vaccination can be influenced not only by the status of the gastrointestinal microbiota but also by various factors related to the environment and vaccine adjuvants (
34). One of the limitations of our study is the small number of animals involved. In future research, we believe that expanding the scope of the study to include probiotic agents commonly used in veterinary practice, a larger sample size, additional immunological parameters, and different vaccination schedules could reveal faster and stronger immune responses following vaccination.
Traditionally, scientific investigations aimed at enhancing immune responses in vaccination have focused on vaccination protocols, adjuvant development, and vaccine strains. However, in recent years, there has been a growing recognition of the importance of evaluating the host’s condition and exploring modifications that can be made to improve vaccine responses (
35). A study on immunity against distemper virus examined standard vaccination procedures and found that the application of acupuncture, a traditional Chinese medicine technique, resulted in nearly twice as high antibody titers compared to the non-applied control group (
35). Similarly, we have observed a positive response in terms of the development of parvovirus antibodies when co-administered with probiotics.
CONCLUSIONImplementing early and continuous vaccination programs incorporating B.
animalis subsp.
lactis and vitamin D can have a substantial impact on the immune system and contribute to the prevention of illnesses by enhancing the production of antibodies against CPV. The findings of our study hold significant relevance for initiatives aimed at utilizing canine vaccination to safeguard and manage the dog population.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThis study was supported by the Faculty of Veterinary Medicine, Aydin Adnan Menderes University. It is part of the first author's Master thesis..
AUTHORS’ CONTRIBUTIONSAll the authors contributed to the design and implementation of the research, analysis of the results, literature search, and writing of the manuscript.

 10.2478/macvetrev-2023-0025
10.2478/macvetrev-2023-0025


