The spread of duck hepatitis virus (DHV) in Egypt with its’ most prevalent genotypes (1 and 3) causes vast economic losses in the duck industry despite the regular vaccination with live attenuated vaccines. However, the use of live-vaccines is considered a potential risk for the non-vaccinated birds due to the viral shedding from vaccinated populations. The current study aimed to evaluate the protective efficacy and safety of two inactivated monovalent and one bivalent vaccines against DHV 1 and 3 genotypes. The inactivated monovalent (DHAV-1, DHAV-3) and bivalent (DHAV-1+3) vaccines were produced by using Montanide ISA 70 oil as an adjuvant. Three groups of 4-week-old ducklings (n=50) were vaccinated with one of the three vaccines, respectively. One group of ducklings was used as negative control (n=25). The immune-response of the vaccinated groups was measured by Virus Neutralization Test and expressed with Neutralizing Indices (NIs). The NIs for the bivalent vaccine group (5.6 and 5.4) were higher compared to the monovalent vaccine groups (5.0 and 4.7). In conclusion, the use of inactivated bivalent DHV vaccine could produce higher protective efficacy compared to the monovalent DHAV-1 and DHAV-3 vaccines.
Duck hepatitis has an economic impact on duck-growing farms because of the high potential for mortality, in case the infection is not controlled, affecting more than 80% of the ducklings under 3 weeks of age (
1). The disease usually occurs in an acute, rapidly spreading, and often fatal viral infection.
Duck hepatitis virus (DHV) is classified into 3 types (I, II, and III) (
2). The DHV type I (DHAV-classical), was first described in the USA in 1949. DHAV has 3 distinguishable genotypes designated as DHAV-1, DHAV-2, and DHAV-3. It is duck hepatitis A virus, member of the genus Avihepatovirus and the family Picornaviridae (
3). DHV types II and III which are antigenically distinct from DHV type I, were recently classified as duck astrovirus 1 and 2 (DAstV-1 and DAstV-2).
DHAV-1 is the most widespread genotype, while DHAV-2 was reported in Taiwan (
4), and DHAV-3 was first characterized in South Korea and China (
1, 5). In addition, no cross-neutralization between DHAV-1 and DHAV-2 has been reported (
4), and limited cross-neutralization between DHAV-1 and DHAV-3 has been reported (
6). Therefore, DHAV-1 vaccine failed to elicit cross protection against DHAV-3 infection, and a live attenuated DHAV-3 vaccine was developed (
7).
Furthermore, simultaneous co-infection by DHAV-1 and DHAV-3 has recently become increasingly frequent in domestic ducks resulting in major economic losses to duck industry in China and Korea (
8, 9), so administration of both DHAV-1 and DHAV-3 vaccines is highly recommended.
The concomitant administration of both vaccines may cause antigen interference and side effects affecting the protective power and safety of the vaccines (
10). Moreover, using two inoculations is cumbersome, expensive, and stressful for the birds, therefore development of an effective and convenient vaccine is urgently needed to prevent parallel infections caused by DHAV-1 and DHAV-3.
In Egypt, DHAV was reported in the late 1970s (
11), then several reports of the disease outbreaks have been documented (
12). Since the expansion of the Egyptian commercial duck farms, DHAV has caused devastating losses and posed a major threat to the commercial duck farms (
12).
Mass vaccination programs for breeder ducks using attenuated vaccines produced from E52 Rispens strain - DHAV-1 vaccinal strain have been implemented in Egypt in order to effectively control the disease (
13, 14). Lately, a new inactivated vaccine using the same strain has been produced by Omar et al. (
15).
So far, DHAV infection remains a vital threat to duck industry in Egypt despite the ongoing official duck vaccination programs (
16, 17). Alternative strategies for developing vaccines against DHAV-1 and DHAV-3 co-infections, by using both genotypes, are urgently needed.
It was hypothesized that the bivalent vaccine would provide a higher one-dose protection against DHAV-1 and DHAV-3 genotypes which can be used in breeder duck farms.
The current study aimed to develop 2 local inactivated monovalent vaccines, DHAV-1 and DHAV-3, and an inactivated bivalent DHAV-1+3 vaccine, and to evaluate their protective efficacy and safety in ducks.
MATERIAL AND METHODSExperimental ducksTwo hundred and twenty, one-day-old Pekin ducklings from a commercial hatchery, were screened for DHV antibodies. The ducklings were housed and grouped for evaluating potency (immunogenicity) and safety of the 3 prepared vaccines. A separate group was used as negative control.
Embryonated chicken eggsSpecific Pathogen Free (SPF) Embryonated Chicken Eggs (ECEs), 9-11 days old, were purchased from Kom Oshim farm for SPF-eggs, El-Fayoum, Egypt. The eggs were used for propagation and titration of DHAV viruses and for vaccine inactivation testing.
Duck viral hepatitis strains a-Duck-hepatitis-A-virus-BH2 (DHAV-1)Duck-hepatitis-A-virus-BH2 (DHAV-1) was a local Egyptian isolate, belonging to DHAV-1, and it was kindly supplied from the National Laboratory for Veterinary Quality Control of Poultry Production (NLQP), Animal Health Research Institute, Giza, Egypt. The strain was named in the gene bank with an accession number of MN873051 and was used for vaccines preparation and as an antigen in Virus Neutralization Test (VNT).
b-Duck-hepatitis-A-virus-BH1 (DHAV-3)Duck-hepatitis-A-virus-BH1 (DHAV-3) was a local Egyptian isolate, belonging to DHAV-3, and it was kindly supplied from the National Laboratory for Veterinary Quality Control of Poultry Production (NLQP), Animal Health Research Institute, Giza, Egypt. The strain was named in the gene bank with an accession number of MN873049 and was used for vaccines preparation and as an antigen in VNT.
Vaccine preparationThe vaccine preparation was done according to the World Organization for Animal Health (OIE) (
18). The local DHAV-1 and DHAV-3 isolates were propagated in 9-day-old SPF ECEs through incubation of inoculated eggs at 37 ºC for 5 days with daily examination. At the end of the examination period, both live and dead embryos were collected and homogenized.
The purified viral homogenates were titrated in 9-day-old SPF ECEs, then inactivated by freshly prepared binary ethylenimine (BEI) with a final concentration of 1% at 37 ºC (
19).
The inactivated viral suspensions were tested for completion of inactivation by intra-allantoic inoculation into ten 9-day-old SPF ECEs, and incubation for 5 days at 37 ºC for 2 serial passages. In each time, eggs were examined for DHV.
The vaccines’ doses were adjusted to contain 10
6 EID
50/strain/dose, and the dose was adjusted to be 0.5 ml for subcutaneous (S/C) inoculation (
15). The adjusted inactivated viral suspensions were adjuvanted with Montanide ISA 70 oil (
19, 20).
Two inactivated monovalent vaccines for DHAV-1 and DHAV-3 strains were prepared, as well as an inactivated bivalent vaccine containing both DHAV-1 and DHAV-3 strains (DHAV-1+3). The 3 vaccines were tested for efficacy, safety, sterility, and completion of inactivation (
18).
Protective efficacy, safety, and sterility evaluation of the vaccinesThe method for efficacy assessment of the inactivated vaccines is described elsewhere (
7, 18, 21). Briefly, subcutaneous vaccination was performed on 50 ducklings, 4-week-old, with 0.5 ml/duckling for each vaccine. Blood samples for VNT were collected 3 days after the vaccination and on weekly basis until the 12
th week post vaccination (WPV).
The safety of the inactivated vaccines was evaluated by vaccination of 15 duckling with 2 doses/duckling for each vaccine. The ducklings were observed for 14 days assessing pathogenic clinical signs and changes in body weight. If none of the vaccinated ducklings showed any adverse reaction, the vaccine was considered safe.
The sterility of the prepared vaccines was evaluated by specific media culturing and incubation determining if there was any potential bacterial and fungal contamination. The inactivation completion testing for each vaccine was performed by intraallantoic vaccine inoculation of ten 9 days old SPF ECE with 2 serial passages. The inoculated eggs were incubated for 5 days at 37 ºC. In each time, eggs were examined for DHV.
Virus neutralization testVNT was carried out according to Woolcock (
22) through tenfold serial dilution of both DHAV viruses, DHAV-1 and DHAV-3, then mixing the dilutions with equal amounts of heat inactivated sera of vaccinated and control duckling, followed by incubation the mixtures at 37 °C for an hour.
Inoculation of the virus and virus-serum mixtures dilutions in 9 days old SPF ECE groups through intra-allantoic route, and incubation at 37 °C for 5 days with daily examination, record of mortalities and chilling of dead eggs. At the end of the incubation period, chilling of all remaining live eggs over night before examination.
The examination of all eggs for DHV lesion and calculation of viruses’ titers in each inoculated group by Reed and Muench (
23). The calculation of the Neutralizing index (NI) for each group by subtracting the titer of each virus-serum group from the titer of virus group.
Ethical disclaimsAll experimental animals in this study weretreated in strict accordance with and adherence to the relevant policies regarding animal handling as mandated under international, national, and/or institutional guidelines for the care of animals, approved by the Research Ethical Committee at the National Research Center, Cairo, Egypt.
RESULTSThe titers of DHAV-1 and DHAV-3 local isolates were 10
8.5 and 10
7.1 EID
50/ml respectively after serial passages in SPF ECEs, and the inactivated isolates were found completely inactivated.
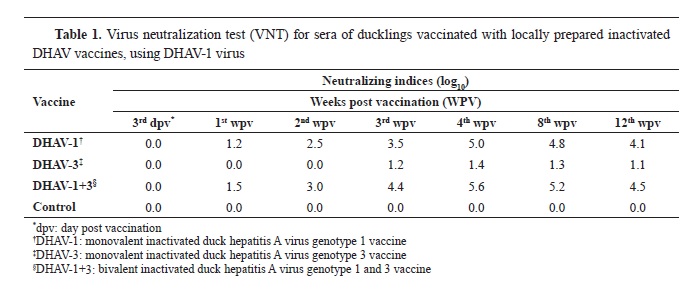
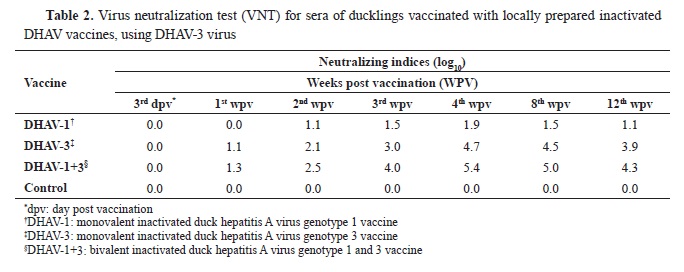
The results of the vaccines’ tests showed that the prepared inactivated monovalent and bivalent DHAV vaccines were completely inactivated, sterile and safe for duck vaccination. The evaluation of immune responses for the vaccines was measured by VNT, and the Neutralization Indices (NIs) for weekly collected serum samples were described in
Tables 1 and
2.
DISCUSSIONIn the past few years, DHV is considered to be a severe threat to the duck breeding industry in Egypt. Mixed infections caused by DHAV-1 and DHAV-3 viruses have become common in domestic ducks. This high level of DHAV outbreaks require effective control measures. Vaccination remains the most effective tool for duck protection against DHAV infections.
Nowadays, live attenuated and inactivated vaccines are available in Egypt for controlling only DHAV-1 infection in ducks (
15). There are no commercial vaccines approved for duck protection against DHAV-3 infection. The study aimed to develop an inactivated vaccine that protects the ducks against both DHAV-1 and DHAV-3 which could be used for limiting the DHAVs problem in Egypt.
Two inactivated monovalent vaccines (DHAV-1 and DHAV-3) and an inactivated bivalent vaccine (DHAV-1+3) were prepared from the 2 locally isolated DHAV-1 and DHAV-3 genotypes (
18). The immunogenicity of the prepared DHAV vaccines was evaluated by measuring the neutralization efficacy through VNT (
Table 1 and
2). The VNT is not only used in detection of maternal immunity antibodies but also it is used in detection of DVH antibodies in all ages. The challenge test is used only in ducks under 6 weeks of age due to ageresistance. For the current study, which included adult commercial ducks, VNT was used instead of the challenge test as described elsewhere (
24). The VNT measurements in this study were expressed as NIs (
21, 25).
Table 1 shows the weekly measured NI means following the vaccination of ducks with the prepared inactivated monovalent and bivalent DHAV vaccines against DHAV-1 virus. The NIs began to increase from the 1
st WPV for the monovalent DHAV-1 and bivalent DHAV-1+3 vaccines (1.2 and 1.5 log
10, respectively), and the peak was reached at the 4
th WPV (5.0 and 5.6 log
10, respectively). The mean NIs began to decrease slightly at the 8
th WPV (4.8 and 5.2 log
10) reaching 4.1 and 4.5 log
10, respectively at the 12
th WPV. The monovalent DHAV-3 vaccine produced poor NIs against DHAV-1 virus with highest value at the 4
th WPV (1.4 log
10). Noticeably, the antibodies produced by the bivalent DHAV-1+3 vaccine gave higher NIs for DHAV-1 virus infection throughout all WPV compared to the monovalent DHAV-1 vaccine (
Table 1). Both vaccines produced protective antibodies after the 2
nd WPV. The monovalent DHAV-3 vaccine failed to produce protective level of antibodies against the local DHAV-1 virus according to OIE (
18).
OIE indicates that if neutralizing antibodies could neutralize >100 LD
50 of DHV infectivity, the vaccine is deemed to be protective (
18). Other studies reported that NI >1.5 log
10 was sufficient to protect ducks against infection with DHAV-1 (
19).
Similarly, the same results were almost shown when the prepared monovalent and bivalent DHAV vaccines were tested against DHAV-3 virus (
Table 2). The monovalent DHAV-1 vaccine produced lower neutralizing antibody titers to DHAV-3 virus reaching the maximum NI (1.9 log
10) at the 4
th WPV. The NI started to decrease from the 8
th WPV (1.5 log
10) until 12
th WPV (1.1 log
10). In the same way, the mean NIs for the monovalent DHAV-3 and the bivalent DHAV-1+3 vaccines against DHAV-3 virus started to increase from the 1
st WPV (1.1 and 1.3 log
10, respectively), then reached a peak at the 4
th WPV (4.7 and 5.4 log
10, respectively). Following this period, it started to decrease slightly from the 8
th WPV (4.5 and 5.0 log
10, respectively) until 12
th WPV (3.9 and 4.3 log
10, respectively). The bivalent DHAV-1+3 vaccine gave higher protective NIs against DHAV-3 virus starting from the 2nd WPV compared to the monovalent DHAV-3 vaccine. In addition, the monovalent DHAV-1 vaccine could produce partial protection to the ducks against DHAV-3 only after the 4
th WPV.
Briefly, the results of this study indicated that the DHAV-1 vaccines, either monovalent or bivalent, are completely protective against DHAV-1 infection and partially protective against DHAV-3 infection only for small period after vaccination with poor neutralizing antibodies (only after 4 WPV).
The DHAV-3 vaccines, either monovalent or bivalent, are also effectively protective for ducks from all ages against DHAV-3 infection but could not protect the ducks against DHAV-1 infection at any age after vaccination. This is compliant with another report (
26) which stated that there was no cross reaction between DHAV-1 and DHAV-3 in the tested duck sera collected from ducks vaccinated with DHAV bivalent vaccine.
Finally, the obtained results indicated that there is no cross immunization between DHAV-1 and DHAV-3. Therefore, an optimal protection could be achieved by including the two DHAV genotypes (1 and 3) (
27).
CONCLUSIONThe locally prepared inactivated bivalent DHAV-1+3 vaccine was effective in producing high antibody titers against the two DHAV genotypes (1 and 3) for prolonged period after vaccination. This suggests that the vaccine may be useful to control DHAV outbreaks caused by DHAV-1 and DHAV-3. Additionally, the inactivated DHV vaccines are much more convenient for use by the farmers because they can be easily stored and transported in chilled state compared to the live vaccines which require storage in frozen state.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThe authors are grateful and thankful to the ARC, CLEVB, VSVRI, NLQP and AHRI for their appreciated cooperation and support.
AUTHORS’ CONTRIBUTIONSDMO applied the experiments and wrote the manuscript. MAA, NMM and HMGA applied the experiments and work drafting. NAM made the work drafting and critically reviewed the manuscript. WAES, NY, SEO and AME followed up the practical work and data analysis. ASA supervised, provided local viral strains and data analysis. MAS supervised and made substantial contribution to the work concept. LMO was supervisor, the owner of the main idea and experimental design, and critically reviewed the manuscript. All authors reviewed and approved the final version of this manuscript for publication.

 10.2478/macvetrev-2024-0010
10.2478/macvetrev-2024-0010

