Infection of Babesia-naïve dogs with
Babesia canis triggers an acute phase reaction (APR) resulting with the development of specific humoral and cellular immune responses. These responses control the infection (
1). Apart from the triad of fever, anorexia, and lethargy, the cytokine storm leads to hypotension (
2), and sometimes even to multiple organ dysfunction and shock (
3). This violent immune reaction is triggered by soluble antigens produced by the sporozoites of
B. canis during their asexual reproduction in the erythrocytes of the dog (
4). The soluble antigens adhere to blood cells and play at least a partial role in the development of thrombocytopenia occuring in all dogs with APR (
1, 2, 3, 4). The severity of APR can be detected by elevated acute phase proteins (APPs), such as serum amyloid A (SAA) and even apolipoprotein A I (ApoA I) (
5, 6).
Leukopenia occurs in about half of the dogs infected with
B. canis (
7) and its severity is related to the intensity of the inflammatory response (
8). Anemia develops in about one third (
9) to two thirds of the patients and, at least in
B. rossi, its severity does not correlate with the degree of parasitemia (
10). Several mechanisms are thought to trigger anemia simultaneously. Secondary immune-mediated hemolysis, erythrophagocytosis, complement activation, and a disturbed balance between oxidants and antioxidants followed by oxidative damage are involved (
11, 12). It could also be speculated that proinflammatory cytokines lead to ineffective erythropoiesis, as has been shown in
Plasmodium spp. which also belongs to the intraerythrocytic protozoa (
13). This hypothesis remains to be proven for the acute form of
B. canis infection.
Clinical recovery of dogs infected with
B. canis after being treated with imdocarb-dipropionate is usually very rapid (
14), but it has been shown that the oxidant/antioxidant imbalance and erythrocyte damage persist for two weeks after the treatment, even though the anemia has been corrected (
12). Few studies have been conducted to investigate the reticulocyte response during the course of the disease and the results are inconclusive (
15, 16, 17, 18). In addition, it is known that erythropoiesis is more common in young animals than in adults (
19). Previous results show that ApoA I is a positive APP in dogs with acute
B. canis infection (
5). ApoA I is important for reverse cholesterol transport, but also has antioxidant properties (
20), which could influence the regenerative response of the erythroid lineage. However, the regenerative response of reticulocytes in young and adult dogs infected with
B. canis has not yet been investigated. Furthermore, it is not known whether apoA I levels are different in young and adult dogs. Two studies have reported a robust reticulocyte response in dogs infected with
B. canis and
B. rossi (
15, 17). In these studies, the reticulocyte count was expressed as a percentage. The most reliable measure for evaluating the efficacy of erythropoiesis is the absolute reticulocyte count. The absolute reticulocyte count is a desirable method to compare the regenerative response, as relative numbers may lead to misinterpretation in anemia classification (
21).
The aim of the present study was to investigate hematologic parameters with emphasis on the reticulocyte count on the day of admission and on day 14 after therapy with imidocarb-dipropionate in young and adult dogs naturally infected with
B. canis. ApoA I levels and protein and lipid profiles were compared between two groups of dogs and at two-time points.
MATERIAL AND METHODSThe study was conducted on 22 privately owned dogs that were naturally infected with
B. canis. The dogs were presented in March and April 2017 at a private veterinary practice “Petrovac” in a suburb of Belgrade (Serbia), which is endemic for vector ticks infected with
B. canis -
Dermacentor reticulatus (
22). An owner consent was obtained for the cases included in this study. The study was also approved by the Ethics Committee of the Faculty of Veterinary Medicine of the University of Belgrade, and the Ministry of Agriculture and Environmental Protection of the Republic of Serbia issued permit No. 323-07-03455/2015-05/3 to conduct the study.
The dogs eligible for this study had: (1) Presence of clinical signs consistent with
Babesia spp. infection in dogs (anorexia, fever, lethargy, pale or icteric mucous membranes, hemoglobinuria); (2) Large babesia forms in erythrocytes on the Romanowsky stained (BioDiff, BioGnost, Zagreb, Croatia) thin blood smears; (3)
B. canis-positive polymerase chain reaction (PCR; Tick/Vector Comprehensive RealPCR Panel Canine, IDEXX Laboratories, Westbrook, Maine).
The dogs were divided into two groups according to their age. One group consisted of 11 young dogs, 4 females and 7 males (median 10, range 8-12 months). The other group consisted of 11 adult dogs, 6 females and 5 males (median 4.5, range 2-7 years). Blood samples were taken on the day of admission and 14 days after the treatment with imidocarb-dipropionate.
The blood samples with ethylenediaminetetraacetic acid (EDTA) (Vacutainer, Becton Dickinson, Franklin Lakes, New Jersey) were used for complete blood count, blood smear preparation, reticulocyte count and PCR, while the samples without anticoagulant were used for serum separation (Vacutainer, Becton Dickinson). The serum samples used in this study for the radioimmunoassay were obtained from the serum surplus after routine biochemical diagnostics. The samples were centrifuged for 10 minutes (1,500 g) and the serum excess was stored at −20 ˚C for up to two months.
Complete blood counts (CBC) were obtained using the Abacus Junior Vet™ hematology analyzer (Diatron, Vienna, Austria) immediately upon the blood collection. Serum concentration of total cholesterol, triglycerides, protein, and albumin was determined using commercial reagents (Elitech™, Puteaux, France) on automated biochemical analyzer Technicon RA-XT™ (Bayer™, Dublin, Ireland).
The number of reticulocytes in the blood was measured using a method called supravital staining with brilliant cresyl blue. To perform this test, equal amounts of EDTA blood and supravital dye solution (1.0% w/v of brilliant cresyl blue) were mixed and left to incubate for 15 minutes at room temperature. The percentage of reticulocytes was determined by examining blood smears under a light microscope at 100x magnification (1000 erythrocytes were counted). The absolute reticulocyte count was calculated by multiplying the reticulocyte percentage by the red blood cell count. The anemia was classified as non-regenerative if the number of reticulocytes was below 65×10
9/L (
23).
The concentration of ApoA I was measured in serum samples using a rabbit polyclonal antibody (0.5 μg per tube) from Santa Cruz Biotechnology, based in Santa Cruz, California. We followed a previously reported method with slight modifications (
24) and measured the ApoA I concentration using radioimmunoassay. The concentration of ApoA I was expressed in arbitrary units (U), as described (
5).
Statistical analysis was done by MedCalc statistical software (version 16.2.1, Ostend, Belgium). To compare vital signs between young and adult dogs infected with
B. canis on the day of presentation, a Mann-Whitney test was utilized. Categorical data were tested with Chi-square test. To test paired samples, repeated measures analysis of variance (ANOVA) was used. Withinsubjects factors were blood count and biochemical parameters on the day of admission and the day 14 after therapy with imidocarb-dipropionate. The between-subjects factor was dogs age (young and adult). A p value of <0.05 was considered significant.
RESULTSUpon admission, the majority of dogs exhibited fever and tachypnea. Heart rate was higher in young dogs compared to adults.
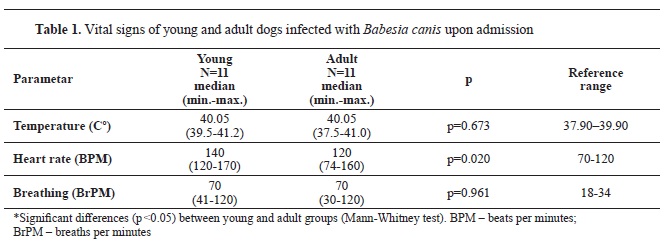
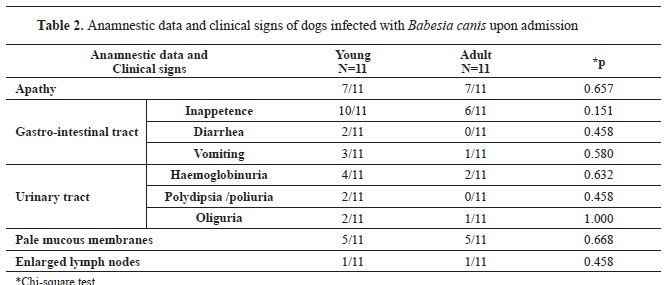
According to the study, young dogs are more likely to experience gastrointestinal signs such as loss of appetite, diarrhea, and vomiting compared to adult dogs. Similarly, urinary tract problems like hemoglobinuria, increased thirst and urination, and reduced urine output were slightly more frequent in young than in adult dogs. However, signs like apathy, pale mucous membranes, and enlarged lymph nodes were equally present in both age groups (
Table 2).
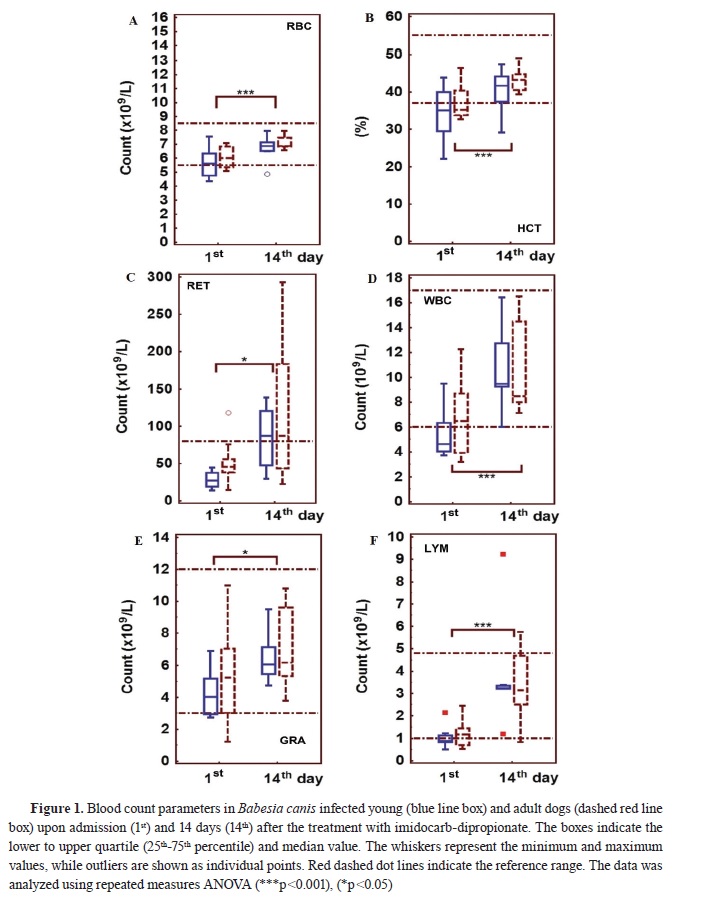
Upon admission, both groups of dogs showed lower counts of red blood cells, reticulocytes, white blood cells, granulocytes, and lymphocytes as compared to day 14 following imidocarbdipropionate treatment (
Fig. 1). There was no significant difference in erythroid lineage parameters between young and adult dogs (
Fig. 1A, B, C). The hematocrit value was below the reference range in both groups upon admission (
Fig. 1B). Additionally, the reticulocyte count was under 65×10
9 /L, but increased on day 14 following treatment (
Fig. 1C). Although there was no statistical difference in the white blood cell counts between young and adult dogs, it is worth noting that the count was below the lower limit in most young dogs.
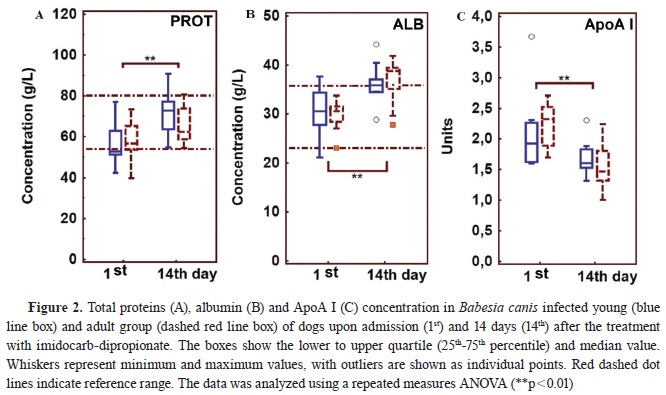
The concentration of total protein and albumin in young and adult dogs was found to be lower at presentation as compared to 14 days after treatment (
Fig. 2A, B). On the other hand, the concentration of ApoA I was higher in both groups of dogs on the day of admission as compared to 14 days after treatment with imidocarb-dipropionate (
Fig. 2C).
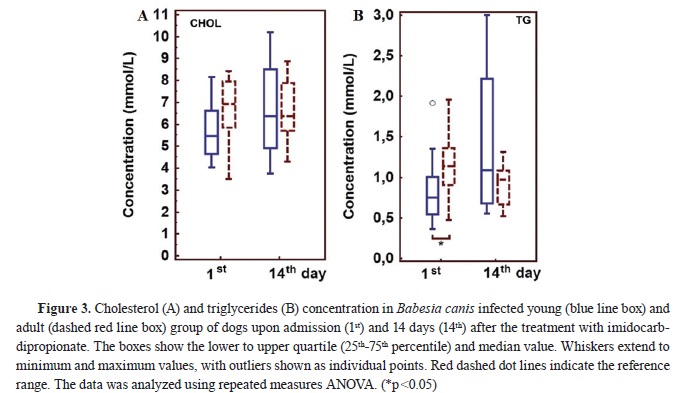
There was no significant difference in cholesterol concentration between young and adult dogs on the first and 14
th day, as shown in Fig. 3A. On the other hand, Fig. 3B indicates that the concentration of triglycerides was significantly lower in young compared to adult dogs on the day of admission.
DISCUSSIONBoth young and adult dogs showed signs of apathy, inappetence, and pale mucous membranes. However, signs indicating digestive and urinary tract involvement were slightly more common in young than in adult dogs. These findings are consistent with previous reports (
25, 26). Specifically, acute
B. canis infection is more common in young dogs (
26) and tends to cause more severe clinical signs (
25).
The low hematology values observed upon admission were found to be similar between young and adult dogs. Even though a significant improvement was noted after 14 days of treatment, there was no difference between the two age groups. However, it should be noted that all hematology parameters tended to be lower in young dogs, both at admission and after treatment, albeit not significantly. This can be attributed to two possible reasons: first, the reference range for erythroid lineage is lower in young dogs up to one year and stabilizes thereafter in the adult range (
27), suggesting that the young dogs in the study have already reached their steady state values after 14 days of therapy; second, young dogs, which have a more vigorous APR, may require more time to establish hematological values that match those before the infection.
Upon admission, anemia was established in both groups of dogs, and it was non-regenerative, as the number of reticulocytes was below 65×10
9/L, which indicates a poor bone marrow response. The leading cause of anemia due to
B. canis infection is hemolysis (
11). Hemolytic anemia is usually regenerative and may even become macrocytic and hypochromic if regeneration is robust (
27). During our study, dogs showed signs of acute phase response (APR) for at least 24 to 48 hours.
Experimental
B. canis infection leads to depression of the erythroid lineage between day 3 and 5 (
1). It is suggested that the decrease in erythroid lineage parameters is still in its early stages, and the reticulocyte response is not accelerated enough. However, it is interesting that erythropoiesis is not more efficiently stimulated in young animals, despite the abundance of growth factors (
19). One possibility could be that proinflammatory cytokines suppress erythropoiesis in both young and adult dogs (
27). Additionally, it is hypothesized that babesia species (other than
B. canis) secrete inhibitory substances that decrease the activity of erythrocyte 5”-nucleotidase, which leads to an accumulation in cytidine monophosphate and inosine 5“-monophosphate, influencing reticulocyte maturation (
28).
B. canis may have the same effect. Hypotension with hemodilution at the onset of infection is one of the mechanisms responsible for the drop in erythroid lineage parameters (
2). After treatment, a marked reticulocyte response was demonstrated by day 8, although the estimation was based on reticulocyte percentages (
15). In our study, regenerative response was still seen 14 days after treatment, with high numbers of reticulocytes in the face of non-anemic dogs. This finding suggests that the erythroid lineage recovers quickly and efficiently in both young and adult dogs after successful treatment.
The concentration of ApoA I is higher in dogs infected with
B. canis compared to healthy ones (
5, 29). It appears that ApoA I functions as a positive APP in
B. canis infection. As ApoA I is involved in reverse cholesterol transport, it was expected that cholesterol concentration would be different at admission compared to 14 days after treatment. However, we were unable to prove this effect, most likely due to the complex metabolic regulation of both ApoA I and cholesterol. Nevertheless, the antioxidant role of ApoA I can be considered protective, as oxidative injury at least partially mediates the hemolytic process (
12). It is noteworthy that the observed effect could not be linked to the current number of reticulocytes as the changes were consistent across the groups and time periods. Additionally, it is interesting to note that the young dogs have a lower concentration of triglycerides than adult dogs. This could be due to a more potent acute phase response with more severe anorexia or a more pronounced effect of the endocrine system on lipid metabolism in young dogs. Specifically, metabolic pathways during anorexia and APR stimulate the breakdown of fats and the production of very low-density lipoproteins in liver cells.
These lipoproteins are the only ones that are rich in triglycerides when anorexia is present (
30). It is likely that young animals have less adipose tissue and produce less VLDL in response to an APR. A rise in total protein levels 14 days after treatment may indicate an effective antibody response, while an increase in albumin may suggest a stable condition after an APR and the initial hemodilution that dogs experience upon admission.
CONCLUSIONAcute
B. canis infection in dogs that develop an APR, is characterized with non-regenerative anemia in young and adult dogs. Fourteen days after successful treatment with imidocarb-dipropionate, anemia was corrected and high reticulocyte count was present. This indicates that the erythroid lineage has responded efficiently to the treatment in young dogs despite the possibility that this group had more severe APR. Among protein and lipid parameters that could indicate difference in severity of APR between young and adult dogs, only triglycerides showed lower values in young dogs. A decrease in ApoA I was observed in both groups of dogs 14 days after treatment, confirming its status as a positive APP in acute
B. canis infection in dogs. Further studies are needed to connect its role in erythroid lineage regeneration.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSWe would like to express our gratitude to Vladimir Radonjic, DVM, TaurunumVet, Belgrade, Serbia for enrolling the patients, and to Ramaswamy Chandrashekar, Toni Mastek, and Phyllis Tyrell from IDEXX Laboratories, Westbrook, Maine, for facilitating PCR diagnostics. This study was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (contract number: 451-03-47/2023-01/200143).
AUTHORS’ CONTRIBUTIONSZM, AIB, VR, JFA made the sample collection, data collection, data analysis, designand drafting the manuscript. ŽBT, LJH, RC performed the radioimmunoassay, PCR and data analysis. MKF revised the manuscript critically for important intellectual content.

 10.2478/macvetrev-2024-0011
10.2478/macvetrev-2024-0011




