Infectious hematopoietic necrosis (IHN) is a common disease in the intensive production of salmonids. The IHN virus (IHNV) was isolated for the first time in North Macedonia in 2018. This study aimed to investigate the distribution and genotype of IHN in farmed rainbow trout and autochthonous salmonid fish in North Macedonia following the first detection. The samples were collected from 47 trout farms. Trout fry with or without clinical signs of IHN were selected as individual samples. Kidney, spleen, and heart were taken from each fish during the dissection. Three pooled samples were collected from each farm. A total of 141 pooled samples were collected: Rainbow trout (Oncorhynchus mykiss) n=127, Macedonian trout (Salmo macedonicus) n=11, and Ohrid trout (Salmo letnica) n=3. The virus was detected in 43 samples (30.50%): rainbow trout (n=40), Macedonian trout (n=2), and Ohrid trout (n=1). There were 18 (38.30%) positive fish farms. The MAKIHNV1 isolate from 2018 (MN641902) and the newly isolated virus shared a similarity of >99 and were placed in clade E-1 of European genogroup E. The IHN has spread throughout the country and is also present in the autochthonous salmonids.
Infectious hematopoietic necrosis virus (IHNV) is an important pathogen that causes a lethal disease in wild and cultured salmonid species. Its high pathogenicity can lead to cumulative mortality of up to 100%, depending on the environmental conditions, fish species, and size (
1). The causative agent is a member of the genus
Novirhabdovirus in the family Rhabdoviridae (
2). Its virion is bullet-shaped, encapsulating the unsegmented and negative-sense single-stranded RNA genome, which encodes five structural proteins, including nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), RNA polymerase (L), and unique non-virion protein (NV) (
3). The surface glycoprotein is the only IHNV protein capable of inducing neutralizing antibodies in the host cells (
2). This protein has a greater genetic diversity than the other viral proteins and is amenable to intensive studies for phylogenetic analysis or for antigen identification for vaccine development (
3).
IHNV was observed for the first time in enzootic areas in the western part of North America in the Pacific salmon (
4, 5). In 1987, IHNV was already introduced in Europe, in France, and Italy; in 1992, it was spread to Germany (
6). As a result, the salmonid aquaculture in France, Italy, and Germany suffered heavy economic losses. In Balkan Peninsula IHNV was detected in Slovenia in 2002 (
7), Croatia in 2007 (
8), and Kosovo in 2011 (
9).
The first report of IHNV in farmed rainbow trout in North Macedonia was in 2018 (
10). The virus was isolated from an asymptomatic rainbow trout fry and was confirmed by virus isolation on EPC cells, qRT-PCR, RT-PCR and sequencing. The Macedonian IHNV isolate is located with Croatian and Slovenian IHNV isolates in the Eur 1 cluster of the European IHNV. However, this isolate is closely related to recent isolates from Germany, Italy and Iran. French isolates may represent the origin of several German IHNV isolates (
11), and the French IHNV genotype has spread through the European countries mainly through the trade of infected fish, fry and eggs (
12). The other countries in the region (Serbia, Bulgaria and Greece) that have implemented the Directive 2006/88: European Union animal health requirements for aquaculture animals and products, have surveillance program for listed aquatic disease, but there have been no positive IHNV reports.
The study aimed to investigate the distribution and genotype of IHNV in farmed rainbow trout and its presence in farmed autochthonous salmonid fish in North Macedonia.
MATERIAL AND METHODSSample collectionThe samples were collected between September 2020 and December 2020 when water temperatures range from 8 and 15 °C. The Food and Veterinary Agency in North Macedonia has been monitoring for IHNV and VHSV since 2015. The monitoring program in 2020 included 47 trout farms distributed in 6 administrative regions of North Macedonia (Eastern, n=6; Northeastern, n=3; Pelagonia, n=6; Polog, n=10; Southwestern, n=16; and Vardar, n=6). The monitoring in 2020 started earlier after one fish farm had reported mortality after buying fingerlings and that triggered the epidemiology investigation. The samples from that farm were selected from the fish with clinical signs. On the other farms, there were no clinical signs, and trout fry were sampled randomly from different locations on the farm. Kidney, spleen, and heart were taken from each fish during the dissection. Three pooled samples (10 fish per pool) were taken from each farm. A total of 141 pooled samples from 47 fish farms were collected consisting of rainbow trout (
Oncorhynchus mykiss) n=127, Macedonian trout (
Salmo macedonicus) n=11, and Ohrid trout (
Salmo letnica) n=3.
The samples were taken on-site at the farm, stored at 4 °C in a transport medium and transported to the Laboratory for Molecular Diagnostics at the Faculty of Veterinary Medicine-Skopje (FVM-S) for testing.
RNA extractionThe genetic material from the samples was isolated using the SaMag24 automated extraction system (Sacace Biotechnologies) and the SaMag Total RNA/DNA extraction kit (Sacace Biotechnologies), according to the manufacturer’s protocol. The aggregates (25 mg) were homogenized in a stainless-steel Eppendorf tube and metal ball for 5 min/22 Hz/min with a MM Mixer Mill 400 (Retsch) and were briefly centrifuged. After initial sample processing, 200 μl of supernatant from all samples was processed for nucleic acid extraction. To optimize the RNA in the PCR reaction, the quantity and purity of the extracted RNA were evaluated using a UV spectrophotometer (NanoDrop 2000C, Thermo Scientific) and the A260/280 ratio was determined.
Virus detectionA highly sensitive RT-qPCR protocol was used to detect IHNV (
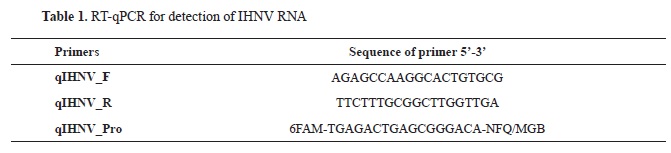
13). The RT-qPCR reaction was performed in QuantStudio5 (Applied Biosystems) using the Path-ID™ Multiplex One-Step RT-PCR Kit (Ambion, Applied Biosystems). The primers used in this study amplified a short sequence in the N gene. The nucleotide sequences of the primers, as well as the method of preparation of the primer mixtures, are given in
Tables 1 and
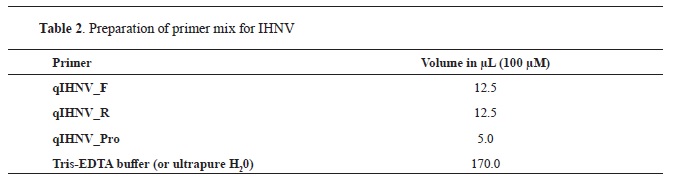
2. The reaction was carried out in a total volume of 22.50 μl, with the following composition of the mixture: 14.25 μl DEPC treated water, 6.25 μl 4x TaqMan Fast Virus 1-Step Master Mix, and 2 μl primer mix for IHNV.
A standard curve was used to check the repeatability and reproducibility of the test using a reference material labelled Aqua I (lyophilized material from rainbow trout from France: AY524121 (abbreviated G-gene), FJ265711 (N-gene). The material was obtained from the EU Reference Laboratory DTU Aqua, Denmark. In the laboratory using this amplification protocol, Aqua I was calibrated at a Ct (threshold cycle) value of 20.8 Ct. The thermal protocol for performing the RT-qPCR reaction was as follows: 50 °C for 2 minutes, 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute.
Five isolates from regions with Ct less than 30 were selected for phylogenetic analyses following the procedure described by Cvetkovikj et al. (
10).
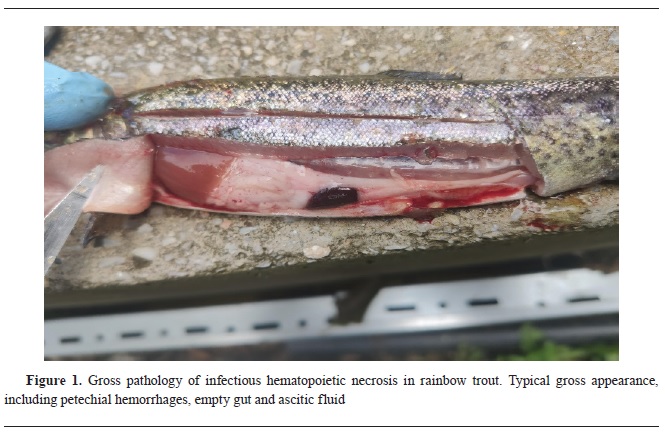
RESULTSIHNV was detected in 30.50% of the tested samples (43/141): 31.49% of the rainbow trout samples (40/127), 18.18% of the Macedonian trout samples (2/11), and 33.33% of the Ohrid trout samples (1/3) were positive for IHNV. In total, 38.30% (18/47) of the fish farms were positive. Clinical signs as: skin’s darkening, pale gills, exophthalmos and a swollen abdomen, pale internal organs, spinal deformities in surviving fish, were observed only in one fish farm with rainbow trout. The fish farm had mortality of 60% in fingerlings (
Fig. 1).
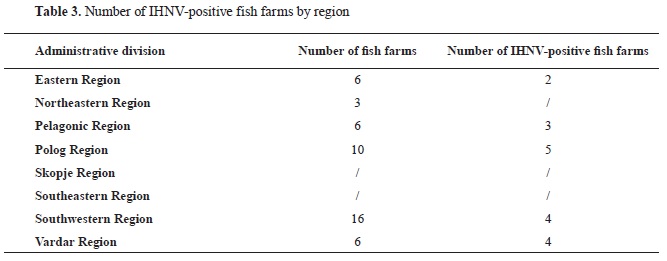
The distribution of positive samples in the administrative regions is shown in
Table 3.
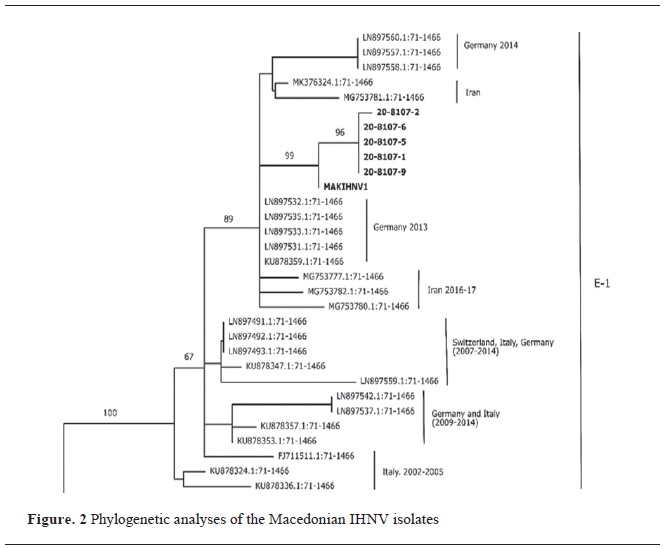
The GenBank BLAST sequence was most similar to the IHNV isolate MAKIHNV1, isolated from North Macedonia in 2018 (MN641902), with a similarity of 99.74% (20-8197-6, 20-8197-5, 20-8197-1 and 20-8197-9) and 99.66% (
Fig. 2). All isolates were placed in clade E-1 of European genogroup E (
10).
DISCUSSIONThis study has revealed that the IHN has spread throughout the country and is also present in a clinical form. The study has also found that the Macedonian trout and the Ohrid trout are susceptible to the infection with the IHNV and that the infection was asymptomatic.
The phylogenetic analyzes classified the isolates in the E–1 clade of the E (European) genogroup together with the previous Macedonian isolate MAKIHNV1 (
10) and together with isolates from continental European countries, including Germany and Italy from 2013 and 2014 and Iran from 2014 to 2017 (
14).
These results indicate that the IHNV in North Macedonia has spread widely across the fish farms. These results and the epidemiology surveying will provide concrete evidence for the management strategy design.
The intensive production, although yielding higher quantities, poses risks regarding disease transmission and compliance with regulatory measures. To address this situation, North Macedonian fish farms must adhere to regulations and guidelines for juvenile fish production. This entails implementing strict biosecurity measures, regularly monitoring fish health, and following appropriate quarantine protocols for imported eggs. Collaboration among industry stakeholders, including fish farmers, regulatory authorities, and hatchery operators, is essential to develop and enforce robust protocols for disease prevention, early detection, and control, particularly concerning IHNV. Proper training and education on biosecurity practices can raise awareness and improve compliance within the rainbow trout farming sector in North Macedonia. To reduce IHNV morbidity and mortality in trout farms, an effective eradication plan, improved biosecurity criteria, and aquaculture health management practices are necessary. Basic hygiene education and training for fish farmers are effective and cost-efficient methods. Disinfection of eggs is crucial to prevent vertical transmission of IHNV (
6). Promoting domestic trout egg production would also contribute to a decrease in their importation from abroad. It is therefore recommended to establish reproduction centers for rainbow trout and to implement program to produce IHNV-resistant strains to minimize the risk of increasing IHNV prevalence in North Macedonia (
15, 16). Additionally, efforts should be focused on developing a vaccination program, which has already been reported in recent years (
17).
The study was conducted over a relatively short period. Conducting longer-term studies would be beneficial to gain a more comprehensive understanding of IHNV dynamics and trends. The study’s small sample size of autochthonous fish may hinder the assessment of IHNV prevalence and genotypic diversity in these native fish populations. Increasing the sample size in future studies would improve the accuracy and reliability of the findings. The study’s primary focus was on fish samples from farmed rainbow trout, potentially neglecting samples from fish in open water or natural environments. This limitation restricts the generalizability of the findings to wild fish populations and their interactions with farmed fish. A more comprehensive understanding of IHNV distribution and genotyping in both farmed and wild fish populations would be provided if were included samples from open-water environments. The study only sequenced five isolates of IHNV. Increasing the number of isolates genotyped would provide a broader range of data and contribute to a more comprehensive understanding of IHNV distribution and genotyping in North Macedonia’s farmed and wild fish populations.
Longer-duration studies, larger sample sizes of autochthonous fish, including samples from open water environments, and genotype analysis on a broader range of samples would enhance our knowledge of IHNV in farmed and wild fish populations in North Macedonia.
CONCLUSIONThis study underscores the prevalence of the infectious hematopoietic necrosis virus (IHNV) in North Macedonia’s rainbow trout farms. It has revealed that the IHN is also present in a clinical form. The Macedonian and Ohrid trout are susceptible to the virus with asymptomatic infection. The presence of the clinical form of the disease highlights the need for robust prevention measures, especially when trading with eggs and fry. Collaboration among stakeholders and implementing strict biosecurity measures, health monitoring, and quarantine protocols are vital for safeguarding the sector. Future research should include more extended studies, larger sample sizes, and diverse sample sources for a comprehensive understanding of IHNV distribution. Expanding the genotyping analysis can enhance our knowledge of IHNV in North Macedonia.
Addressing these challenges is crucial for effective IHNV management and the sustainability of rainbow trout farming in North Macedonia.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThe authors would like to acknowledge the Faculty of Veterinary Medicine-Skopje, Ss. Cyril and Methodius University in Skopje, the Food and Veterinary agency of North Macedonia and the European Union Reference Laboratory (EURL) for Fish and Crustacean Diseases for sharing their logistical, intellectual, and research infrastructure for this study.
AUTHORS’ CONTRIBUTIONAT conceptualized the study, collected and analyzed the samples and wrote the manuscript. ID and KK supervised the sample analysis. ZP carried out the sample analysis. MN was included in the hypothesis formulation and manuscript writing. LR and DB participated in the collection of the samples. AG was coordinating fieldwork. NV and AC contributed to the practical performance of this research. AC supervised the study and manuscript writing. All authors have revised and approved the final version of the manuscript.

 10.2478/macvetrev-2024-0013
10.2478/macvetrev-2024-0013




